Cognitive Effects of Three β-Adrenoceptor Acting Drugs in Healthy Volunteers and Patients with Parkinson's Disease
- PMID: 39213090
- PMCID: PMC11380312
- DOI: 10.3233/JPD-240039
Cognitive Effects of Three β-Adrenoceptor Acting Drugs in Healthy Volunteers and Patients with Parkinson's Disease
Abstract
Background: Noradrenergic signaling declines in Parkinson's disease (PD) following locus coeruleus neurodegeneration. Epidemiologic studies demonstrate that β-acting drugs slow PD progression.
Objective: The primary objective was to compare the safety and effects of 3 β-adrenoceptor (β-AR) acting drugs on central nervous system (CNS) function after a single dose in healthy volunteers (HVs) and evaluate the effects of multiple doses of β-AR acting drugs in HVs and PD-patients.
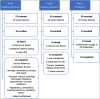
Methods: In Part A, HVs received single doses of 32 mg salbutamol, 160μg clenbuterol, 60 mg pindolol and placebo administered in a randomized, 4-way cross-over study. In Part B (randomized cross-over) and Part C (parallel, 2:1 randomized), placebo and/or clenbuterol (20μg on Day 1, 40μg on Day 2, 80μg on Days 3-7) were administered. CNS functions were assessed using the NeuroCart test battery, including pupillometry, adaptive tracking and recall tests.
Results: Twenty-seven HVs and 12 PD-patients completed the study. Clenbuterol improved and pindolol reduced the adaptive tracking and immediate verbal recall performance. Clenbuterol and salbutamol increased and pindolol decreased pupil-to-iris ratios. Clenbuterol was selected for Parts B and C. In Part B, clenbuterol significantly increased performance in adaptive tracking with a tendency toward improved performance in immediate and delayed verbal recall. In Part C trends toward improved performance in immediate and delayed verbal recall were observed in PD-patients. Typical cardiovascular peripheral β2-AR effects were observed with clenbuterol.
Conclusions: This study demonstrates the pro-cognitive effects of clenbuterol in HVs with similar trends in PD-patients. The mechanism of action is likely activation of β2-ARs in the CNS.
Keywords: Parkinson’s disease; adrenergic beta-agonist; central nervous system agents; disease progression; electroencephalography; pharmacology; phase 1 clinical trial.
Plain language summary
Aims and Purpose of the Research:This research aimed to explore how three different drugs affect brain function. These drugs are salbutamol, clenbuterol, and pindolol and work in the brain by stimulating specific brain cells that can improve aspects like memory and coordination. The main question was to see how safe these drugs were and how they impact the brain function after one dose in healthy people, and after multiple doses in both healthy people and those with Parkinson’s disease.Background of the Research:Parkinson’s disease is a condition where brain cells start to die, which affects different areas of the brain, including movement function, as well as memory and attention. This research matters because finding drugs that affect the brain function could improve the lives of people with Parkinson’s disease.Methods and Research Design:The study was conducted in three parts. In the first part, healthy volunteers took one dose of each of the three drugs— salbutamol, clenbuterol, and pindolol— as well as a placebo (a harmless pill that has no effect). The researchers tested the participants’ brain functions using various tasks including memory tests and eye response measurements. In the second and third part, healthy people and people with Parkinson’s disease took the drug that performed best in healthy volunteers for seven days.Results and Importance:In the first part, a single dose of clenbuterol was safe and improved memory and attentions tasks in healthy people, and therefore was chosen for further testing in the second and third part. In these parts, multiple doses of clenbuterol were safe and helped improve memory and attention tasks in healthy people, with similar positive trends seen in people with Parkinson’s disease. The study suggests that clenbuterol might help improve brain function by activating specific receptors in the brain.These results are important because they suggest that clenbuterol could be a potential treatment to help improve brain function in people with Parkinson’s disease. However, more research is needed to fully understand its effects and to confirm these findings.
Conflict of interest statement
This study was funded by CuraSen Therapeutics, Inc. PE, LB, SP, LM, BvK, EvB, EK, PK, GJ are investigators, statisticians and site personnel at the clinical investigative site for this study; EB is a paid consultant to CuraSen; RM, AF and GV are employees of CuraSen Therapeutics, Inc.
Figures


References
-
- Routledge CandMarsden CA. Adrenaline in the CNS: in vivo evidence for a functional pathway innervating the hypothalamus. Neuropharmacology 1987; 26: 823–830. - PubMed
-
- Cash R, Ruberg M, Raisman R, et al. Adrenergic receptors in Parkinson’s disease. Brain Res 1984; 322: 269–275. - PubMed
-
- Beitz JM. Parkinson’s disease: a review. Front Biosci (Schol Ed) 2014; 6: 65–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

