Immunization with germ line-targeting SOSIP trimers elicits broadly neutralizing antibody precursors in infant macaques
- PMID: 39213340
- PMCID: PMC12302664
- DOI: 10.1126/sciimmunol.adm7097
Immunization with germ line-targeting SOSIP trimers elicits broadly neutralizing antibody precursors in infant macaques
Abstract
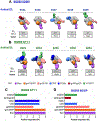
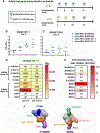
Adolescents are a growing population of people living with HIV. The period between weaning and sexual debut presents a low-risk window for HIV acquisition, making early childhood an ideal time for implementing an immunization regimen. Because the elicitation of broadly neutralizing antibodies (bnAbs) is critical for an effective HIV vaccine, our goal was to assess the ability of a bnAb B cell lineage-designed HIV envelope SOSIP (protein stabilized by a disulfide bond between gp120-gp41-named "SOS"-and an isoleucine-to-proline point mutation-named "IP"-at residue 559) to induce precursor CD4 binding site (CD4bs)-targeting bnAbs in early life. Infant rhesus macaques received either a BG505 SOSIP, based on the infant BG505 transmitted/founder virus, or the CD4bs germ line-targeting BG505 SOSIP GT1.1 (n = 5 per group). Although both strategies induced durable, high-magnitude plasma autologous virus neutralization responses, only GT1.1-immunized infants (n = 3 of 5) exhibited VRC01-like CD4bs bnAb precursor development. Thus, a multidose immunization regimen with bnAb lineage-designed SOSIPs shows promise for inducing early B cell responses with the potential to mature into protective HIV bnAbs before sexual debut.
Conflict of interest statement
Figures





References
-
- UNICEF, “Adolescent HIV prevention,” https://data.unicef.org/topic/hivaids/adolescents-young-people/.
-
- Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell CA, Eroshkin AM, Guenaga J, Kaushik K, Kulp DW, Liu J, McCoy LE, Oom AL, Ozorowski G, Post KW, Sharma SK, Steichen JM, de Taeye SW, Tokatlian T, Torrents de la Pena A, Butera ST, LaBranche CC, Montefiori DC, Silvestri G, Wilson IA, Irvine DJ, Sanders RW, Schief WR, Ward AB, Wyatt RT, Barouch DH, Crotty S, Burton DR, Elicitation of robust Tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46, 1073–1088.e1076 (2017). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

