Neoadjuvant 177Lu-DOTATATE for non-functioning pancreatic neuroendocrine tumours (NEOLUPANET): multicentre phase II study
- PMID: 39213395
- PMCID: PMC11364141
- DOI: 10.1093/bjs/znae178
Neoadjuvant 177Lu-DOTATATE for non-functioning pancreatic neuroendocrine tumours (NEOLUPANET): multicentre phase II study
Abstract
Background: Resection of non-functioning pancreatic neuroendocrine tumours (NF-PanNETs) is curative in most patients. The potential benefits of neoadjuvant treatments have, however, never been explored. The primary aim of this study was to evaluate the safety of neoadjuvant 177Lu-labelled DOTA0-octreotate (177Lu-DOTATATE) followed by surgery in patients with NF-PanNETs.
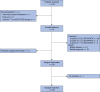
Methods: NEOLUPANET was a multicentre, single-arm, phase II trial of patients with sporadic, resectable or potentially resectable NF-PanNETs at high-risk of recurrence; those with positive 68Ga-labelled DOTA PET were eligible. All patients were candidates for neoadjuvant 177Lu-DOTATATE followed by surgery. A sample size of 30 patients was calculated to test postoperative complication rates against predefined cut-offs. The primary endpoint was safety, reflected by postoperative morbidity and mortality within 90 days. Secondary endpoints included rate of objective radiological response and quality of life.
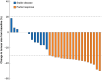
Results: From March 2020 to February 2023, 31 patients were enrolled, of whom 26 completed 4 cycles of 177Lu-DOTATATE. A partial radiological response was observed in 18 of 31 patients, and 13 patients had stable disease. Disease progression was not observed. Twenty-four R0 resections and 4 R1 resections were performed in 29 patients who underwent surgery. One tumour was unresectable owing to vascular involvement. There was no postoperative death. Postoperative complications occurred in 21 of 29 patients. Severe complications were observed in seven patients. Quality of life remained stable after 177Lu-DOTATATE and decreased after surgery.
Conclusion: Neoadjuvant treatment with 177Lu-DOTATATE is safe and effective for patients with NF-PanNETs.
Plain language summary
Surgical removal of non-functioning pancreatic neuroendocrine tumours (NF-PanNETs) can often cure patients. However, it is not known whether treating patients before surgery provides additional benefits. The main aim of the study was to investigate safety of treatment called peptide receptor radionuclide therapy with 177Lu-labelled DOTA0-octreotate (177Lu-DOTATATE) before surgery in patients with NF-PanNETs. The NEOLUPANET study was conducted from March 2020 to February 2023 across multiple centres. Patients with NF-PanNETs that were at high risk of recurrence after surgery were included. All patients received 177Lu-DOTATATE before surgery. The main measure of success was safety, reflected by complications or death within 90 days after surgery. The study enrolled 31 patients and 26 completed four cycles of 177Lu-DOTATATE. Tumours shrank partially in 18 patients, and in 13 patients the tumour remained the same. No tumours increased in size. The entire tumour was removed in 28 of 29 patients. No patients died, but 72% had some complications (severe in 7 patients). The study found that using 177Lu-DOTATATE before surgery is safe and useful for treating patients with NF-PanNETs.
© The Author(s) 2024. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures
References
-
- Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 2015;42:5–19 - PubMed
-
- Paganelli G, Sansovini M, Nicolini S, Grassi I, Ibrahim T, Amadori E et al. 177Lu-PRRT in advanced gastrointestinal neuroendocrine tumors: 10-year follow-up of the IRST phase II prospective study. Eur J Nucl Med Mol Imaging 2021;48:152–160 - PubMed
-
- Brabander T, Van Der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, De Herder WW et al. Long-term efficacy, survival, and safety of [177Lu-DOTA0, Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 2017;23:4617–4624 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical