Local radiation enhances systemic CAR T-cell efficacy by augmenting antigen crosspresentation and T-cell infiltration
- PMID: 39213422
- PMCID: PMC11700247
- DOI: 10.1182/bloodadvances.2024012599
Local radiation enhances systemic CAR T-cell efficacy by augmenting antigen crosspresentation and T-cell infiltration
Abstract
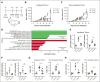
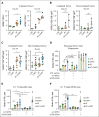
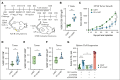
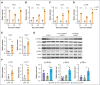
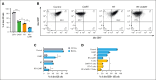
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 (CART-19) represents a significant advance in the treatment of patients with relapsed or refractory CD19+ B-cell lymphomas. However, a significant portion of patients either relapse or fail to respond. Moreover, many patients have symptomatic disease, requiring bridging radiation therapy (RT) during the period of CAR T-cell manufacturing. To investigate the impact of 1 to 2 fractions of low-dose RT on CART-19 treatment response, we developed a mouse model using A20 lymphoma cells for CART-19 therapy. We found that low-dose fractionated RT had a positive effect on generating abscopal systemic antitumor responses beyond the irradiated site. The combination of RT with CART-19 therapy resulted in additive effects on tumor growth in irradiated masses. Notably, a significant additional increase in antitumor effect was observed in nonirradiated tumors. Mechanistically, our results validate activation of the cyclic guanosine adenosine synthetase/stimulator of interferon genes pathway, tumor-associated antigen crosspriming, and elicitation of epitope spreading. Collectively, our findings suggest that RT may serve as an optimal priming and bridging modality for CAR T-cell therapy, overcoming treatment resistance and improving clinical outcomes in patients with CD19+ hematologic malignancies.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.A.C. reports funding from Genmab, Genentech/Roche, AstraZeneca, CARGO, Juno Therapeutics, Novartis, Lymphoma Research Foundation, and Kite Pharma; and advisory board from AstraZeneca and Beigene. S.J.S. reports research funding from TG Therapeutics, Incyte, Adaptive Biotechnologies, Pharmacyclics, Merck, Genmab, Genentech/Roche, AstraZeneca, Cargo, AbbVie, and Lymphoma Research Foundation; consultancy fees and research funding from Genentech/Roche, Juno Therapeutics, and AbbVie; consultancy fees from Tessa Therapeutics, Loxo Oncology, BeiGene, Alimera Sciences, Acerta Pharma/AstraZeneca, and Nordic Nanovector; consultancy fees, honoraria, patents and royalties, and research funding from Novartis; consultancy fees, honoraria, and research funding from Celgene; and advisory board fees from AstraZeneca and BeiGene. The remaining authors declare no competing financial interests.
Figures



References
-
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

