Rab27a GTPase and its effector Myosin Va are host factors required for efficient Oropouche virus cell egress
- PMID: 39213446
- PMCID: PMC11392402
- DOI: 10.1371/journal.ppat.1012504
Rab27a GTPase and its effector Myosin Va are host factors required for efficient Oropouche virus cell egress
Abstract
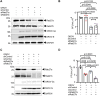
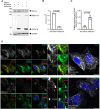
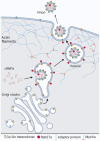
Oropouche fever, a debilitating illness common in South America, is caused by Oropouche virus (OROV), an arbovirus. OROV belongs to the Peribunyaviridae family, a large group of RNA viruses. Little is known about the biology of Peribunyaviridae in host cells, especially assembly and egress processes. Our research reveals that the small GTPase Rab27a mediates intracellular transport of OROV induced compartments and viral release from infected cells. We show that Rab27a interacts with OROV glycoproteins and colocalizes with OROV during late phases of the infection cycle. Moreover, Rab27a activity is required for OROV trafficking to the cell periphery and efficient release of infectious particles. Consistently, depleting Rab27a's downstream effector, Myosin Va, or inhibiting actin polymerization also hinders OROV compartments targeting to the cell periphery and infectious viral particle egress. These data indicate that OROV hijacks Rab27a activity for intracellular transport and cell externalization. Understanding these crucial mechanisms of OROV's replication cycle may offer potential targets for therapeutic interventions and aid in controlling the spread of Oropouche fever.
Copyright: © 2024 Concha et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Barbosa NS, Concha JO, daSilva LLP, Rezaei N. Bunyavirus. Encyclopedia of Infection and Immunity. Oxford: Elsevier; 2022. p. 207–18.
-
- Pinheiro FP, Travassos da Rosa AP, Travassos da Rosa JF, Ishak R, Freitas RB, Gomes ML, et al. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am J Trop Med Hyg. 1981;30(1):149–60. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

