MET exon 14 skipping mutations in non-small-cell lung cancer: real-world data from the Italian biomarker ATLAS database
- PMID: 39214048
- PMCID: PMC11402035
- DOI: 10.1016/j.esmoop.2024.103680
MET exon 14 skipping mutations in non-small-cell lung cancer: real-world data from the Italian biomarker ATLAS database
Abstract
Background: Mesenchymal-epithelial transition (MET) exon 14 (METex14) skipping mutation is a rare alteration in non-small-cell lung cancer (NSCLC), occurring in about 3%-4% of cases. Here we report disease and patient characteristics, and efficacy and tolerability of MET inhibitors among advanced METex14 NSCLC patients from the Italian real-world registry ATLAS.
Materials and methods: Clinical-pathological and molecular data, and treatment efficacy/tolerability outcomes were retrospectively collected from the ATLAS registry.
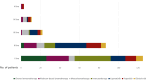
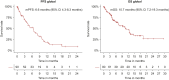
Results: From July 2020 to July 2023 a total of 146 METex14 advanced NSCLC patients were included across 27 Italian centers. Median age was 74 years, and most patients were male (52%), with an Eastern Cooperative Oncology Group performance status < 2 (72%) and adenocarcinoma subtype (83%). One hundred and twenty-five out of 146 (86%) patients received at least one line of systemic anticancer therapy. Fifty-six (38%) were treated with capmatinib and 34 (23%) with tepotinib. 29% and 52% of them received targeted treatment in the first and second line, respectively. In the cohort of patients treated with MET inhibitors, the response rate (RR) was 37% (33% in previously treated patients and 46% in treatment-naïve) with a disease control rate of 62%. With a median follow-up of 10.8 months, progression-free survival was 6.6 months [95% confidence interval (CI) 4.3-8.3 months] and overall survival was 10.7 months (95% CI 7.2-19.3 months). In patients with measurable brain metastases (17 cases), the intracranial RR was 41%. Grade ≥3 treatment-related adverse events (TRAEs) occurred in 12% of patients with grade 3 peripheral edema in 7% of cases. A fatal adverse reaction occurred in one patient due to pneumonitis. TRAEs-related dose reduction and discontinuation were reported in 6% and 8% of cases, respectively.
Conclusion: Capmatinib and tepotinib represent an effective treatment option in NSCLC patients with METex14. Real-world efficacy outcomes are worse than those reported in prospective clinical trials. Their activity is more pronounced in the treatment-naïve population, suggesting that this is the right setting in the management of patients with METex14.
Keywords: MET exon 14 skipping; capmatinib; non-small-cell lung cancer; targeted therapy; tepotinib.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Frampton G.M., Ali S.M., Rosenzweig M., et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. - PubMed
-
- Remon J., Hendriks L.E.L., Mountzios G., et al. MET alterations in NSCLC-current perspectives and future challenges. J Thorac Oncol. 2023;18(4):419–435. - PubMed
-
- Tong J.H., Yeung S.F., Chan A.W.H., et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22:3048–3056. - PubMed
-
- Kron A., Scheffler M., Heydt C., et al. Genetic heterogeneity of MET-aberrant NSCLC and its impact on the outcome of immunotherapy. J Thorac Oncol. 2021;16(4):572–582. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

