Cellular Uptake of Phase-Separating Peptide Coacervates
- PMID: 39214144
- PMCID: PMC11558145
- DOI: 10.1002/advs.202402652
Cellular Uptake of Phase-Separating Peptide Coacervates
Abstract
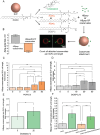
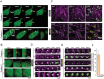
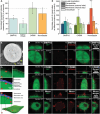
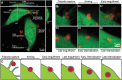
Peptide coacervates self-assembling via liquid-liquid phase separation are appealing intracellular delivery vehicles of macromolecular therapeutics (proteins, DNA, mRNA) owing to their non-cytotoxicity, high encapsulation capacity, and efficient cellular uptake. However, the mechanisms by which these viscoelastic droplets cross the cellular membranes remain unknown. Here, using multimodal imaging, data analytics, and biochemical inhibition assays, we identify the key steps by which droplets enter the cell. We find that the uptake follows a non-canonical pathway and instead integrates essential features of macropinocytosis and phagocytosis, namely active remodeling of the actin cytoskeleton and appearance of filopodia-like protrusions. Experiments using giant unilamellar vesicles show that the coacervates attach to the bounding membrane in a charge- and cholesterol-dependent manner but do not breach the lipid bilayer barrier. Cell uptake in the presence of small molecule inhibitors - interfering with actin and tubulin polymerization - confirm the active role of cytoskeleton remodeling, most prominently evident in electron microscopy imaging. These findings suggest a peculiar internalization mechanism for viscoelastic, glassy coacervate droplets combining features of non-specific uptake of fluids by macropinocytosis and particulate uptake of phagocytosis. The broad implications of this study will enable to enhance the efficacy and utility of coacervate-based strategies for intracellular delivery of macromolecular therapeutics.
Keywords: cell uptake; drug delivery; macropinocytosis; peptide coacervates; phagocytosis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors AM and YS have filed a US patent on HB
Figures



References
-
- Sun Y., Lim Z. W., Guo Q., Yu J., Miserez A., MRS Bull. 2020, 45, 1039.
-
- Liu J., Spruijt E., Miserez A., Langer R., Nat. Rev. Mater. 2023, 8, 139.
-
- Lim Z. W., Ping Y., Miserez A., Bioconjugate Chem. 2018, 29, 2176. - PubMed
-
- Lim Z. W., Varma V. B., Ramanujan R. V., Miserez A., Acta Biomater. 2020, 110, 221. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
