TGF-β signaling promotes eosinophil activation in inflammatory responses
- PMID: 39214980
- PMCID: PMC11364686
- DOI: 10.1038/s41419-024-07029-2
TGF-β signaling promotes eosinophil activation in inflammatory responses
Abstract
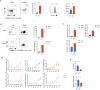
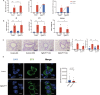
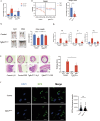
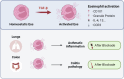
Eosinophils, traditionally associated with allergic phenomena, play a pivotal role in inflammatory responses. Despite accumulating evidence suggesting their pro-inflammatory function upon activation, the underlying mechanisms governing eosinophil activation remain incompletely characterized. In this study, we investigate the local activation of pulmonary and colon eosinophils within the inflammatory microenvironment. Leveraging transcriptional sequencing, we identify TGF-β as a putative regulator of eosinophil activation, leading to the secretion of granule proteins, including peroxidase. Genetic deletion of TGF-β receptors on eosinophils resulted in the inhibition of peroxidase synthesis, affirming the significance of TGF-β signaling in eosinophil activation. Using models of HDM-induced asthma and DSS-induced colitis, we demonstrate the indispensability of TGF-β-driven eosinophil activation in both disease contexts. Notably, while TGF-β signaling did not significantly influence asthmatic inflammation, its knockout conferred protection against experimental colitis. This study delineates a distinct pattern of eosinophil activation within inflammatory responses, highlighting the pivotal role of TGF-β signaling in regulating eosinophil behavior. These findings deepen our comprehension of eosinophil-related pathophysiology and may pave the way for targeted therapeutic approaches in allergic and inflammatory diseases.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- U22A20265/National Natural Science Foundation of China (National Science Foundation of China)
- 82270023/National Natural Science Foundation of China (National Science Foundation of China)
- 82000028/National Natural Science Foundation of China (National Science Foundation of China)
- 82370030/National Natural Science Foundation of China (National Science Foundation of China)
- 2023C03067/Science and Technology Department of Zhejiang Province
LinkOut - more resources
Full Text Sources
Medical

