Network-based prioritization and validation of regulators of vascular smooth muscle cell proliferation in disease
- PMID: 39215134
- PMCID: PMC11182749
- DOI: 10.1038/s44161-024-00474-4
Network-based prioritization and validation of regulators of vascular smooth muscle cell proliferation in disease
Abstract
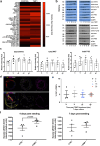
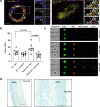
Aberrant vascular smooth muscle cell (VSMC) homeostasis and proliferation characterize vascular diseases causing heart attack and stroke. Here we elucidate molecular determinants governing VSMC proliferation by reconstructing gene regulatory networks from single-cell transcriptomics and epigenetic profiling. We detect widespread activation of enhancers at disease-relevant loci in proliferation-predisposed VSMCs. We compared gene regulatory network rewiring between injury-responsive and nonresponsive VSMCs, which suggested shared transcription factors but differing target loci between VSMC states. Through in silico perturbation analysis, we identified and prioritized previously unrecognized regulators of proliferation, including RUNX1 and TIMP1. Moreover, we showed that the pioneer transcription factor RUNX1 increased VSMC responsiveness and that TIMP1 feeds back to promote VSMC proliferation through CD74-mediated STAT3 signaling. Both RUNX1 and the TIMP1-CD74 axis were expressed in human VSMCs, showing low levels in normal arteries and increased expression in disease, suggesting clinical relevance and potential as vascular disease targets.
© 2024. The Author(s).
Conflict of interest statement
M.S. is a shareholder of Enhanc3D Genomics Ltd. H.F.J. is a key opinion leader for Novo Nordisk A/S. The other authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
- RG/20/2/34763/BHF_/British Heart Foundation/United Kingdom
- RE/13/6/30180/BHF_/British Heart Foundation/United Kingdom
- FS/15/38/31516/British Heart Foundation (BHF)
- MC-A652-5QA20/RCUK | MRC | Medical Research Foundation
- RE/13/ 6/30180/British Heart Foundation (BHF)
- RG/19/9/34655/BHF_/British Heart Foundation/United Kingdom
- FS/15/62/32032/BHF_/British Heart Foundation/United Kingdom
- PG/16/11/32021/BHF_/British Heart Foundation/United Kingdom
- RE/18/1/34212/British Heart Foundation (BHF)
- PG/19/6/34153/BHF_/British Heart Foundation/United Kingdom
- FS/15/38/31516/BHF_/British Heart Foundation/United Kingdom
- RG/16/13/32609/BHF_/British Heart Foundation/United Kingdom
- PG/18/73/34059/BHF_/British Heart Foundation/United Kingdom
- RM/13/3/30159/BHF_/British Heart Foundation/United Kingdom
- CH/2000003/12800/BHF_/British Heart Foundation/United Kingdom
- MC_UP_1605/3/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- CH/2000003/12800/British Heart Foundation (BHF)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
