Unidirectional association of clonal hematopoiesis with atherosclerosis development
- PMID: 39215150
- PMCID: PMC11485253
- DOI: 10.1038/s41591-024-03213-1
Unidirectional association of clonal hematopoiesis with atherosclerosis development
Abstract
Clonal hematopoiesis, a condition in which acquired somatic mutations in hematopoietic stem cells lead to the outgrowth of a mutant hematopoietic clone, is associated with a higher risk of hematological cancer and a growing list of nonhematological disorders, most notably atherosclerosis and associated cardiovascular disease. However, whether accelerated atherosclerosis is a cause or a consequence of clonal hematopoiesis remains a matter of debate. Some studies support a direct contribution of certain clonal hematopoiesis-related mutations to atherosclerosis via exacerbation of inflammatory responses, whereas others suggest that clonal hematopoiesis is a symptom rather than a cause of atherosclerosis, as atherosclerosis or related traits may accelerate the expansion of mutant hematopoietic clones. Here we combine high-sensitivity DNA sequencing in blood and noninvasive vascular imaging to investigate the interplay between clonal hematopoiesis and atherosclerosis in a longitudinal cohort of healthy middle-aged individuals. We found that the presence of a clonal hematopoiesis-related mutation confers an increased risk of developing de novo femoral atherosclerosis over a 6-year period, whereas neither the presence nor the extent of atherosclerosis affects mutant cell expansion during this timeframe. These findings indicate that clonal hematopoiesis unidirectionally promotes atherosclerosis, which should help translate the growing understanding of this condition into strategies for the prevention of atherosclerotic cardiovascular disease in individuals exhibiting clonal hematopoiesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures

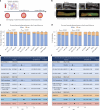

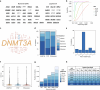
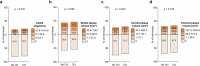


Comment in
-
Clonal haematopoiesis: an emerging causal risk factor for atherosclerotic CVD.Nat Rev Cardiol. 2024 Nov;21(11):741. doi: 10.1038/s41569-024-01081-3. Nat Rev Cardiol. 2024. PMID: 39289541 No abstract available.
References
-
- Mustjoki, S. & Young, N. S. Somatic mutations in “benign” disease. N. Engl. J. Med.384, 2039–2052 (2021). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

