Molecular classification of ovarian high-grade serous/endometrioid carcinomas through multi-omics analysis: JGOG3025-TR2 study
- PMID: 39215190
- PMCID: PMC11473812
- DOI: 10.1038/s41416-024-02837-x
Molecular classification of ovarian high-grade serous/endometrioid carcinomas through multi-omics analysis: JGOG3025-TR2 study
Abstract
Background: Considerable interobserver variability exists in diagnosis of ovarian high-grade endometrioid carcinoma (HGEC) and high-grade serous carcinoma (HGSC) due to histopathological similarities. While homologous recombination deficiency (HRD) correlates with drug sensitivity in HGSC, the molecular features of HGEC are unclear.
Methods: Fresh-frozen samples from 15 ovarian HGECs and 274 ovarian HGSCs in the JGOG-TR2 cohort were submitted to targeted DNA sequencing, RNA sequencing, DNA methylation array, and SNP array. We additionally analyzed 555 ovarian HGSCs from TCGA-OV and 287 endometrial high-grade carcinomas from TCGA-UCEC.
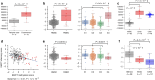
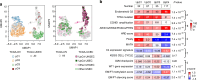
Results: Unsupervised clustering using copy number signatures identified four distinct tumor groups (C1, C2, C3 and C4). C1 (n = 41) showed CCNE1 amplification and poor survival. C2 (n = 160) and C3 (n = 59) showed high BRCA1/2 alteration frequency with low and moderate ploidy, respectively. C4 (n = 22) was characterized by favorable outcome, higher HGEC proportion, no BRCA1/2 alteration or CCNE1 amplification, and low levels of HRD score, ploidy, intra-tumoral heterogeneity, cell proliferation rate, and WT1 gene expression. Notably, C4 exhibited a normal endometrium-like DNA methylation profile, thus, defined as "HGEC-type" tumors, which were also identified in TCGA-OV and TCGA-UCEC.
Conclusions: Ovarian "HGEC-type" tumors present a non-HRD status, favorable prognosis, and endometrial differentiation, possibly constituting a subset of clinically diagnosed HGSCs.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
NM received a research grant from AstraZeneca. NM received lecture fees from AstraZeneca, Takeda Pharmaceutical, Eisai, MSD and Chugai Pharmaceutical, and is an outside director of Takara Bio. TB received lecture fees from AstraZeneca. KY received lecture fees and a research grant from AstraZeneca. There are no other competing interests related to this paper.
Figures


References
-
- Nakai H, Higashi T, Kakuwa T, Matsumura N. Trends in gynecologic cancer in Japan: incidence from 1980 to 2019 and mortality from 1981 to 2021. Int J Clin Oncol. 2024;29:363–71. - PubMed
-
- Yamaguchi K, Mandai M, Oura T, Matsumura N, Hamanishi J, Baba T, et al. Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene. 2010;29:1741–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

