Exploratory pharmacodynamics and efficacy of PF-06817024 in a Phase 1 study of patients with chronic rhinosinusitis and atopic dermatitis
- PMID: 39215351
- PMCID: PMC11365161
- DOI: 10.1186/s13223-024-00894-8
Exploratory pharmacodynamics and efficacy of PF-06817024 in a Phase 1 study of patients with chronic rhinosinusitis and atopic dermatitis
Abstract
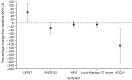
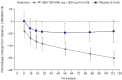
PF-06817024 is a humanized antibody against interleukin-33 that has the potential to inhibit type 2 inflammation. An exploratory analysis of the pharmacodynamics and clinical effects of single and repeat doses of PF-06817024 was assessed in patients with chronic rhinosinusitis with nasal polyps (CRSwNP) and patients with moderate-to-severe atopic dermatitis (AD), respectively, as part of a Phase 1, first-in-human study. Rhinosinusitis symptoms were improved, and nasal polyps were decreased in size following treatment with PF-06817024 in patients with CRSwNP. In patients with AD, PF-06817024, in aggregate, reduced disease severity and improved symptoms, as demonstrated by greater percentage decrease from baseline in Eczema Area and Severity Index (EASI) scores and reduced pruritus numerical rating scores, compared with placebo. The efficacy in AD appeared to be bimodal with a sub-group of participants exhibiting high levels of improvement (EASI75 and EASI90) for a sustained period of time after dosing. In patients with CRSwNP, a consistent trend of decrease in eosinophil levels was observed in the PF-06817024 group, compared with placebo. Further research would be needed to confirm the clinical benefit and safety of PF-06817024 as a treatment for allergic diseases. Trial registration ClinicalTrials.gov, NCT02743871. Registered 15 April 2016, https://clinicaltrials.gov/study/NCT02743871?term=NCT02743871&rank=1 .
Keywords: Anti-IL-33 antibody; Atopic dermatitis; Biomarkers; Chronic rhinosinusitis with nasal polyps; Efficacy; Pharmacodynamics; Phase 1.
© 2024. The Author(s).
Conflict of interest statement
All authors are employees of and hold stock in Pfizer Inc.
Figures
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

