Promotion of endoplasmic reticulum retrotranslocation by overexpression of E3 ubiquitin-protein ligase synoviolin 1 reduces endoplasmic reticulum stress and preserves cone photoreceptors in cyclic nucleotide-gated channel deficiency
- PMID: 39215566
- PMCID: PMC11419579
- DOI: 10.1096/fj.202400198R
Promotion of endoplasmic reticulum retrotranslocation by overexpression of E3 ubiquitin-protein ligase synoviolin 1 reduces endoplasmic reticulum stress and preserves cone photoreceptors in cyclic nucleotide-gated channel deficiency
Abstract
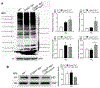
Cone photoreceptor cyclic nucleotide-gated (CNG) channels play an essential role in phototransduction and cellular Ca2+ homeostasis. Mutations in genes encoding the channel subunits CNGA3 and CNGB3 are associated with achromatopsia, progressive cone dystrophy, and early-onset macular degeneration. Cone loss in patients with achromatopsia and cone dystrophy associated with CNG channel mutations has been documented by optical coherence tomography and in mouse models of CNG channel deficiency. Cone death in CNG channel-deficient retinas involves endoplasmic reticulum (ER) stress-associated apoptosis, dysregulation of cellular/ER Ca2+ homeostasis, impaired protein folding/processing, and impaired ER-associated degradation (ERAD). The E3 ubiquitin-protein ligase synoviolin 1 (SYVN1) is the primary component of the SYVN1/SEL1L ER retrotranslocon responsible for ERAD. Previous studies have shown that manipulations that protect cones and reduce ER stress/cone death in CNG channel deficiency, such as increasing ER Ca2+ preservation or treatment with an ER chaperone, increase the expression of SYVN1 and other components of the ER retrotranslocon. The present work investigated the effects of SYVN1 overexpression. Intraocular injection of AAV5-IRBP/GNAT2-Syvn1 resulted in overexpression of SYVN1 in cones of CNG channel-deficient mice. Following treatment, cone density in Cnga3-/- mice was significantly increased, compared with untreated controls, outer segment localization of cone opsin was improved, and ER stress/apoptotic cell death was reduced. Overexpression of SYVN1 also led to increased expression levels of the retrotranslocon components, degradation in ER protein 1 (DERL1), ERAD E3 ligase adaptor subunit (SEL1L), and homocysteine inducible ER protein with ubiquitin-like domain 1 (HERPUD1). Moreover, overexpression of SYVN1 likely enhanced protein ubiquitination/proteasome degradation in CNG channel-deficient retinas. This study demonstrates the role of SYVN1/ERAD in cone preservation in CNG channel deficiency and supports the strategy of promoting ERAD for cone protection.
Keywords: CNG channel; ER stress; ERAD; SYVN1; cone photoreceptors; retinal degeneration.
© 2024 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Disclosures
All authors declare that they have no conflict of interest.
Figures





References
-
- Kohl S, Baumann B, Broghammer M, Jagle H, Sieving P, Kellner U, Spegal R, Anastasi M, Zrenner E, Sharpe LT, and Wissinger B (2000) Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mol Genet 9, 2107–2116 - PubMed
-
- Thiadens AA, Roosing S, Collin RW, van Moll-Ramirez N, van Lith-Verhoeven JJ, van Schooneveld MJ, den Hollander AI, van den Born LI, Hoyng CB, Cremers FP, and Klaver CC Comprehensive analysis of the achromatopsia genes CNGA3 and CNGB3 in progressive cone dystrophy. Ophthalmology 117, 825–830 e821 - PubMed
-
- Wissinger B, Gamer D, Jagle H, Giorda R, Marx T, Mayer S, Tippmann S, Broghammer M, Jurklies B, Rosenberg T, Jacobson SG, Sener EC, Tatlipinar S, Hoyng CB, Castellan C, Bitoun P, Andreasson S, Rudolph G, Kellner U, Lorenz B, Wolff G, Verellen-Dumoulin C, Schwartz M, Cremers FP, Apfelstedt-Sylla E, Zrenner E, Salati R, Sharpe LT, and Kohl S (2001) CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet 69, 722–737 - PMC - PubMed
-
- Thiadens AA, Slingerland NW, Roosing S, van Schooneveld MJ, van Lith-Verhoeven JJ, van Moll-Ramirez N, van den Born LI, Hoyng CB, Cremers FP, and Klaver CC (2009) Genetic etiology and clinical consequences of complete and incomplete achromatopsia. Ophthalmology 116, 1984–1989 e1981 - PubMed
-
- Nishiguchi KM, Sandberg MA, Gorji N, Berson EL, and Dryja TP (2005) Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Hum Mutat 25, 248–258 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

