Malondialdehyde-specific natural IgM inhibit NETosis triggered by culprit site-derived extracellular vesicles from myocardial infarction patients
- PMID: 39215577
- PMCID: PMC11887544
- DOI: 10.1093/eurheartj/ehae584
Malondialdehyde-specific natural IgM inhibit NETosis triggered by culprit site-derived extracellular vesicles from myocardial infarction patients
Abstract
Background and aims: Neutrophil extracellular traps (NETs) trigger atherothrombosis during acute myocardial infarction (AMI), but mechanisms of induction remain unclear. Levels of extracellular vesicles (EV) carrying oxidation-specific epitopes (OSE), which are targeted by specific natural immunoglobulin M (IgM), are increased at the culprit site in AMI. This study investigated EV as inducers of NETosis and assessed the inhibitory effect of natural anti-OSE-IgM in this process.
Methods: Blood from the culprit and peripheral site of ST-segment elevation myocardial infarction (STEMI) patients (n = 28) was collected, and myocardial function assessed by cardiac magnetic resonance imaging (cMRI) 4 ± 2 days and 195 ± 15 days post-AMI. Extracellular vesicles were isolated from patient plasma and cell culture supernatants for neutrophil stimulation in vitro and in vivo, in the presence of a malondialdehyde (MDA)-specific IgM or an isotype control. NETosis and neutrophil functions were assessed via enzyme-linked immunosorbent assay and fluorescence microscopy. Pharmacological inhibitors were used to map signalling pathways. Neutrophil extracellular trap markers and anti-OSE-IgM were measured by ELISA.
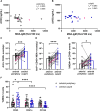
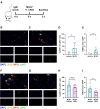
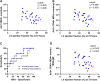
Results: CD45+ MDA+ EV and NET markers were elevated at the culprit site. Extracellular vesicles induced neutrophil activation and NET formation via TLR4 and PAD4, and mice injected with EV showed increased NETosis. Malondialdehyde-specific IgM levels were inversely associated with citH3 in STEMI patient blood. An MDA-specific IgM inhibited EV-induced NET release in vitro and in vivo. CD45+ MDA+ EV concentrations inversely correlated with left ventricular ejection fraction post-AMI.
Conclusions: Culprit site-derived EV induce NETosis, while MDA-specific natural IgM inhibit this effect, potentially impacting outcome after AMI.
Keywords: Acute myocardial infarction; Extracellular vesicles; Natural IgM; Neutrophil extracellular traps; Oxidation-specific epitopes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

