Brain-penetrant complement inhibition mitigates neurodegeneration in an Alzheimer's disease mouse model
- PMID: 39215579
- PMCID: PMC11884734
- DOI: 10.1093/brain/awae278
Brain-penetrant complement inhibition mitigates neurodegeneration in an Alzheimer's disease mouse model
Abstract
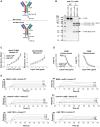
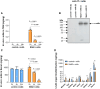
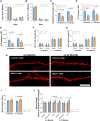
Complement activation is implicated in driving brain inflammation, self-cell damage and progression of injury in Alzheimer's disease and other neurodegenerative diseases. Here, we investigate the impact of brain delivery of a complement-blocking antibody on neurodegeneration in an Alzheimer's mouse model. We engineered a brain-penetrant recombinant antibody targeting the pro-inflammatory membrane attack complex. Systemic administration of this antibody in APPNL-G-F mice reduced brain levels of complement activation products, demonstrating successful brain entry and target engagement. Prolonged treatment decreased synapse loss, amyloid burden and brain inflammatory cytokine levels, concomitant with cognitive improvement compared to controls. These results underscore the potential of brain-penetrant complement-inhibiting drugs as promising therapeutics, targeting downstream of amyloid plaques in Alzheimer's disease.
Keywords: blood–brain barrier; drug delivery; mouse model; neuroinflammation; therapy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report competing interests.
Figures

References
-
- Gupta GL, Samant NP. Current druggable targets for therapeutic control of Alzheimer's disease. Contemp Clin Trials. 2021;109:106549. - PubMed
-
- Imbimbo BP, Watling M. What have we learned from past failures of investigational drugs for Alzheimer's disease? Expert Opin Investig Drugs. 2021;30:1175–1182. - PubMed
-
- Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306–318. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

