Low-Dose Triple-Pill vs Standard-Care Protocols for Hypertension Treatment in Nigeria: A Randomized Clinical Trial
- PMID: 39215620
- PMCID: PMC11366076
- DOI: 10.1001/jama.2024.18080
Low-Dose Triple-Pill vs Standard-Care Protocols for Hypertension Treatment in Nigeria: A Randomized Clinical Trial
Abstract
Importance: With the high burden of hypertension in sub-Saharan Africa, there is a need for effective, safe and scalable treatment strategies.
Objective: To compare, among Black African adults, the effectiveness and safety of a novel low-dose triple-pill protocol compared with a standard-care protocol for blood pressure lowering.
Design and setting: Randomized, parallel-group, open-label, multicenter trial conducted in public hospital-based family medicine clinics in Nigeria.
Participants: Black African adults with uncontrolled hypertension (≥140/90 mm Hg) who were untreated or receiving a single blood pressure-lowering drug.
Interventions: Participants were randomly allocated to low-dose triple-pill or standard-care protocols. The triple-pill protocol involved a novel combination of telmisartan, amlodipine, and indapamide in triple one-quarter, one-half, and standard doses (ie, 10/1.25/0.625 mg, 20/2.5/1.25 mg, and 40/5/2.5 mg), with accelerated up-titration. The standard-care protocol was the Nigeria hypertension treatment protocol starting with amlodipine (5 mg).
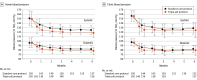
Main outcomes and measures: The primary effectiveness outcome was the reduction in home mean systolic blood pressure, and the primary safety outcome was discontinuation of trial treatment due to adverse events, both from randomization to month 6.
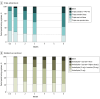
Results: The first participant was randomized on July 19, 2022, and the last follow-up visit was on July 18, 2024. Among 300 randomized participants (54% female; mean age, 52 years; baseline mean home blood pressure, 151/97 mm Hg; and clinic blood pressure, 156/97 mm Hg), 273 (91%) completed the trial. At month 6, mean home systolic blood pressure was on average 31 mm Hg (95% CI, 28 to 33 mm Hg) lower in the triple-pill protocol group and 26 mm Hg (95% CI, 22 to 28 mm Hg) lower in the standard-care protocol group (adjusted difference, -5.8 mm Hg [95% CI, -8.0 to -3.6]; P < .001]). At month 6, clinic blood pressure control (<140/90 mm Hg) was 82% vs 72% (risk difference, 10% [95% CI, -2% to 20%]) and home blood pressure control (<130/80 mm Hg) was 62% vs 28% (risk difference, 33% [95% CI, 22% to 44%]) in the triple-pill compared with the standard-care protocol group; these were 2 of 21 prespecified secondary effectiveness end points. No participants discontinued trial treatment due to adverse events.
Conclusions and relevance: Among Black African adults with uncontrolled hypertension, a low-dose triple-pill protocol achieved better blood pressure lowering and control with good tolerability compared with the standard-care protocol.
Trial registration: Pan African Clinical Trials Registry Identifier: PACTR202107579572114.
Conflict of interest statement
Figures

Comment in
-
Is a Low-Dose Triple-Drug Combination Pill Protocol the Answer for Hypertension Control in Sub-Saharan Africa?JAMA. 2024 Oct 1;332(13):1057-1058. doi: 10.1001/jama.2024.18166. JAMA. 2024. PMID: 39215617 No abstract available.
References
-
- Parati G, Lackland DT, Campbell NRC, et al. ; World Hypertension league . How to improve awareness, treatment, and control of hypertension in Africa, and how to reduce its consequences: a call to action from the World Hypertension League. Hypertension. 2022;79(9):1949-1961. doi:10.1161/HYPERTENSIONAHA.121.18884 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

