Activation of Wnt/β-catenin signaling is critical for the tumorigenesis of choroid plexus
- PMID: 39215664
- PMCID: PMC11726344
- DOI: 10.1093/neuonc/noae176
Activation of Wnt/β-catenin signaling is critical for the tumorigenesis of choroid plexus
Abstract
Background: The choroid plexus (ChP) is the secretory epithelial structure located in the brain ventricles. Choroid plexus tumors (CPTs) are rare neoplasms predominantly occurring in young patients with intensified malignancy in children. CPT treatment is hindered by insufficient knowledge of tumor pathology and the limited availability of valid models.
Methods: Genomic and transcriptomic data from CPT patients were analyzed to identify the putative pathological pathway. Cellular and molecular techniques were employed to validate bioinformatic results in CPT patient samples. Pharmacologic inhibition of Wnt/β-catenin signaling was assessed in CPT cells. Cell-based assays of ChP cell lines were performed following CRISPR-Cas9-derived knockout and overexpression of Wnt/β-catenin pathway genes. A 3D CPT model was generated through CRISPR-Cas9-derived knockout of APC.
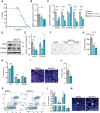
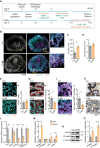
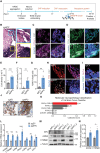
Results: We discovered that Wnt/β-catenin signaling is activated in human CPTs, likely as a consequence of large-scale chromosomal instability events of the CPT genomes. We demonstrated that CPT-derived cells depend on autocrine Wnt/β-catenin signaling for survival. Constitutive Wnt/β-catenin pathway activation, either through knockout of the negative regulator APC or overexpression of the ligand WNT3A, induced tumorigenic properties in ChP 2D in vitro models. Increased activation of the Wnt/β-catenin pathway in ChP organoids, through treatment with a potent GSK3β inhibitor, reduced the differentiation of mature ChP epithelial cells. Remarkably, the depletion of APC was sufficient to induce the oncogenic transformation of ChP organoids.
Conclusions: Our research identifies Wnt/β-catenin signaling as a critical driver of CPT tumorigenesis and provides the first 3D in vitro model for future pathological and therapeutic studies of CPT.
Keywords: APC; Wnt signaling; brain tumor; choroid plexus organoid; rare childhood cancer.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures



References
-
- Lafay-Cousin L, Keene D, Carret AS, et al. Choroid plexus tumors in children less than 36 months: The Canadian Pediatric Brain Tumor Consortium (CPBTC) experience. Childs Nerv Syst. 2011;27(2):259–264. - PubMed
-
- McCall T, Binning M, Blumenthal DT, Jensen RL.. Variations of disseminated choroid plexus papilloma: 2 case reports and a review of the literature. Surg Neurol. 2006;66(1):62–7; discussion 67. - PubMed
-
- Cannon DM, Mohindra P, Gondi V, Kruser TJ, Kozak KR.. Choroid plexus tumor epidemiology and outcomes: Implications for surgical and radiotherapeutic management. J Neurooncol. 2015;121(1):151–157. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

