Collagen IV deficiency causes hypertrophic remodeling and endothelium-dependent hyperpolarization in small vessel disease with intracerebral hemorrhage
- PMID: 39216230
- PMCID: PMC11402910
- DOI: 10.1016/j.ebiom.2024.105315
Collagen IV deficiency causes hypertrophic remodeling and endothelium-dependent hyperpolarization in small vessel disease with intracerebral hemorrhage
Abstract
Background: Genetic variants in COL4A1 and COL4A2 (encoding collagen IV alpha chain 1/2) occur in genetic and sporadic forms of cerebral small vessel disease (CSVD), a leading cause of stroke, dementia and intracerebral haemorrhage (ICH). However, the molecular mechanisms of CSVD with ICH and COL4A1/COL4A2 variants remain obscure.
Methods: Vascular function and molecular investigations in mice with a Col4a1 missense mutation and heterozygous Col4a2 knock-out mice were combined with analysis of human brain endothelial cells harboring COL4A1/COL4A2 mutations, and brain tissue of patients with sporadic CSVD with ICH.
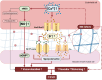
Findings: Col4a1 missense mutations cause early-onset CSVD independent of hypertension, with enhanced vasodilation of small arteries due to endothelial dysfunction, vascular wall thickening and reduced stiffness. Mechanistically, the early-onset dysregulated endothelium-dependent hyperpolarization (EDH) is due to reduced collagen IV levels with elevated activity and levels of endothelial Ca2+-sensitive K+ channels. This results in vasodilation via the Na/K pump in vascular smooth muscle cells. Our data support this endothelial dysfunction preceding development of CSVD-associated ICH is due to increased cytoplasmic Ca2+ levels in endothelial cells. Moreover, cerebral blood vessels of patients with sporadic CSVD show genotype-dependent mechanisms with wall thickening and lower collagen IV levels in those harboring common non-coding COL4A1/COL4A2 risk alleles.
Interpretation: COL4A1/COL4A2 variants act in genetic and sporadic CSVD with ICH via dysregulated EDH, and altered vascular wall thickness and biomechanics due to lower collagen IV levels and/or mutant collagen IV secretion. These data highlight EDH and collagen IV levels as potential treatment targets.
Funding: MRC, Wellcome Trust, BHF.
Keywords: Basement membrane; Cerebrovascular disease; Collagen; Endothelial dysfunction; Small vessel disease; Stroke.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests CDA reports sponsored research support from the American Heart Association (18SFRN34250007 and 21SFRN812095) and Bayer AG, and consulting with ApoPharma, outside the scope of the current work. RA-SS reports grants outside the submitted work from BHF, Chief Scientist Office of the Scottish Government, and National Institutes of Health Research Health Technology Assessment programme paid to The University of Edinburgh, consultancy income paid to The University from Recursion Pharmaceuticals, and reimbursement for endpoint adjudication paid to The University of Edinburgh from NovoNordisk. TVA reports he serves on the grants committee of DEBRA UK and was honorary Treasurer for the British Society of Matrix Biology (2016–2022).
Figures




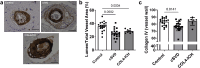
References
-
- Wardlaw J.M., Smith C., Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(7):684–696. - PubMed
-
- Dean A., Van Agtmael T. In: The collagen superfamily and collagenopathies. Ruggiero F., editor. Springer International Publishing; Cham: 2021. Collagen IV-related diseases and therapies; pp. 143–197.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

