Human milk microbiota, oligosaccharide profiles, and infant gut microbiome in preterm infants diagnosed with necrotizing enterocolitis
- PMID: 39216480
- PMCID: PMC11524953
- DOI: 10.1016/j.xcrm.2024.101708
Human milk microbiota, oligosaccharide profiles, and infant gut microbiome in preterm infants diagnosed with necrotizing enterocolitis
Abstract
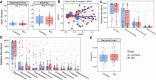
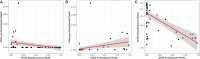
Necrotizing enterocolitis (NEC) is a severe intestinal disease of very preterm infants with mother's own milk (MOM) providing protection, but the contribution of the MOM microbiota to NEC risk has not been explored. Here, we analyze MOM of 110 preterm infants (48 NEC, 62 control) in a cross-sectional study. Breast milk contains viable bacteria, but there is no significant difference in MOM microbiota between NEC and controls. Integrative analysis between MOM microbiota, human milk oligosaccharides (HMOs), and the infant gut microbiota shows positive correlations only between Acinetobacter in the infant gut and Acinetobacter and Staphylococcus in MOM. This study suggests that NEC protection from MOM is not modulated through the MOM microbiota. Thus, "'restoring" the MOM microbiota in donor human milk is unlikely to reduce NEC, and emphasis should instead focus on increasing fresh maternal human milk intake and researching different therapies for NEC prevention.
Keywords: gut microbiome; infant microbiome; microbiota; milk microbiome; multi-omics; necrotizing enterocolitis; preterm infant.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.B. is the UC San Diego Chair of Collaborative Human Milk Research, endowed by the Family Larsson-Rosenquist Foundation (FLRF), Switzerland. L.B. is a co-inventor on patent applications related to the use of HMOs in preventing NEC and other inflammatory diseases. N.D.E. and J.E.B. report grants to their institutions from Prolacta Bioscience US and Danone Early Life Nutrition. N.D.E. declares lecture honoraria donated to charity from Nestlé Nutrition Institute. C.J.S. declares lecture honoraria from Nestlé Nutrition Institute.
Figures


References
-
- Stewart C.J., Embleton N.D., Marrs E.C.L., Smith D.P., Nelson A., Abdulkadir B., Skeath T., Petrosino J.F., Perry J.D., Berrington J.E., Cummings S.P. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome. 2016;4:67. doi: 10.1186/s40168-016-0216-8. - DOI - PMC - PubMed
-
- Battersby C., Longford N., Mandalia S., Costeloe K., Modi N., UK Neonatal Collaborative Necrotising Enterocolitis UKNC-NEC study group Incidence and enteral feed antecedents of severe neonatal necrotising enterocolitis across neonatal networks in England, 2012-13: a whole-population surveillance study. Lancet Gastroenterol. Hepatol. 2017;2:43–51. doi: 10.1016/s2468-1253(16)30117-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

