The GLP-1 medicines semaglutide and tirzepatide do not alter disease-related pathology, behaviour or cognitive function in 5XFAD and APP/PS1 mice
- PMID: 39216535
- PMCID: PMC11408156
- DOI: 10.1016/j.molmet.2024.102019
The GLP-1 medicines semaglutide and tirzepatide do not alter disease-related pathology, behaviour or cognitive function in 5XFAD and APP/PS1 mice
Abstract
Objective: The development of glucagon-like peptide-1 receptor (GLP-1R) agonists for the treatment of type 2 diabetes and obesity has been accompanied by evidence for anti-inflammatory and cytoprotective actions in the heart, blood vessels, kidney, and brain. Whether GLP-1R agonists might be useful clinically for attenuating deterioration of cognitive dysfunction and reducing the progression of Alzheimer's disease remains uncertain.
Methods: Here we evaluated the actions of semaglutide and tirzepatide, clinically distinct GLP-1 medicines, in two mouse models of neurodegeneration.
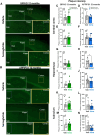
Results: Semaglutide reduced body weight and improved glucose tolerance in 12-month-old male and female 5XFAD and APP/PS1 mice, consistent with pharmacological engagement of the GLP-1R. Nevertheless, amyloid plaque density was not different in the cerebral cortex, hippocampus, or subiculum of semaglutide-treated 12-month-old 5XFAD and APP/PS1 mice. IBA1 and GFAP expression were increased in the hippocampus of 5XFAD and APP/PS1 mice but were not reduced by semaglutide. Moreover, parameters of neurobehavioral and cognitive function evaluated using Open Field testing or the Morris water maze were not improved following treatment with semaglutide. To explore whether incretin therapies might be more effective in younger mice, we studied semaglutide and tirzepatide action in 6-month-old male and female 5XFAD mice. Neither semaglutide nor tirzepatide modified the extent of plaque accumulation, hippocampal IBA1+ or GFAP+ cells, or parameters of neurobehavioral testing, despite improving glucose tolerance and reducing body weight. mRNA biomarkers of inflammation and neurodegeneration were increased in the hippocampus of male and female 5XFAD mice but were not reduced after treatment with semaglutide or tirzepatide.
Conclusions: Collectively, these findings reveal preservation of the metabolic actions of two GLP-1 medicines, semaglutide and tirzepatide, yet inability to detect improvement in structural and functional parameters of neurodegeneration in two mouse models of Alzheimer's disease.
Keywords: Alzheimer's; Diabetes; GLP-1; Glucagon-like peptides; Inflammation; Neurodegeneration; Obesity.
Copyright © 2024 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Dr. Drucker has served as a consultant or speaker within the past 12 months to Altimmune, Amgen, AstraZeneca Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk Inc., Pfizer Inc. and Zealand Pharma Inc. Laurie Baggio is currently an employee of Avalere Health. None of the authors have anything else to declare.
Figures







References
-
- Campbell J.E., Drucker D.J. Pharmacology physiology and mechanisms of incretin hormone action. Cell Metabol. 2013;17(4):819–837. - PubMed
-
- Drucker D.J. Prevention of cardiorenal complications in people with type 2 diabetes and obesity. Cell Metabol. 2024;36(2):338–353. - PubMed
-
- Kosiborod M.N., Abildstrom S.Z., Borlaug B.A., Butler J., Rasmussen S., Davies M., et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–1084. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

