Myeloperoxidase-ANCA IgG induces different forms of small vessel vasculitis based on type of synergistic immune stimuli
- PMID: 39216658
- PMCID: PMC12124227
- DOI: 10.1016/j.kint.2024.08.022
Myeloperoxidase-ANCA IgG induces different forms of small vessel vasculitis based on type of synergistic immune stimuli
Abstract
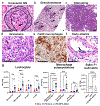
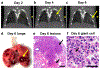
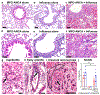
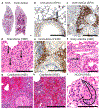
Anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis has diverse patterns of injury including microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Necrotizing and crescentic glomerulonephritis (NCGN) occurs in all syndromes and as renal limited vasculitis (RLV). Single-dose intravenous ANCA IgG-specific for mouse myeloperoxidase (MPO) causes RLV in mice. Although multiple mouse models have elucidated ANCA-IgG induced necrotizing and crescentic glomerulonephritis (NCGN), pathogenesis of ANCA-induced granulomatosis and vasculitis outside the kidney has not been clarified. To investigate this, we used intravenous MPO-ANCA IgG in the same strain of mice to induce different patterns of lung disease mirroring patients with granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Repeated intravenous MPO-ANCA IgG induced GPA with NCGN, lung capillaritis, arteritis and granulomatosis. Lung leukocyte phenotypes were evaluated by immunohistochemical image analysis and by flow cytometry. ANCA lung capillaritis and microabscesses began within one day and evolved into granulomas in under seven days. Influenza plus single-dose MPO-ANCA IgG induced MPA with NCGN, lung capillaritis and arteritis, but no granulomatosis. Allergic airway disease caused by house dust mites or ovalbumin plus single-dose intravenous MPO-ANCA IgG induced EGPA with eosinophilic bronchiolitis, NCGN, capillaritis, arteritis, and granulomatosis. Thus, our study shows that the occurrence and pattern of lung lesions are determined by the same ANCA IgG accompanied by different synergistic immune factors.
Keywords: ANCA; crescentic glomerulonephritis; granulomatosis; polyangiitis; vasculitis.
Copyright © 2024 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure
The authors have no relationships/activities/interests that conflict with the content of our manuscript.
Figures





References
-
- Godman GC, and Churg J. Wegener’s granulomatosis: pathology and review of the literature. AMA Arch Pathol. 1954;58(6):533–53. - PubMed
-
- van der Woude FJ, van der Woude FJ, Rasmussen N, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1(8426):425–9. - PubMed
-
- Falk RJ, and Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318(25):1651–7. - PubMed
-
- Guillevin L, Noel LH, Pourrat J, et al. Antineutrophil cytoplasm antibodies in systemic polyarteritis nodosa with and without hepatitis B virus infection and Churg-Strauss syndrome--62 patients. J Rheumatol. 1993;20(8):1345–9. - PubMed
-
- Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64(10):3452–62. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

