Randomised Trial of No, Short-term, or Long-term Androgen Deprivation Therapy with Postoperative Radiotherapy After Radical Prostatectomy: Results from the Three-way Comparison of RADICALS-HD (NCT00541047)
- PMID: 39217077
- PMCID: PMC7617288
- DOI: 10.1016/j.eururo.2024.07.026
Randomised Trial of No, Short-term, or Long-term Androgen Deprivation Therapy with Postoperative Radiotherapy After Radical Prostatectomy: Results from the Three-way Comparison of RADICALS-HD (NCT00541047)
Abstract
Background and objective: The use and duration of androgen deprivation therapy (ADT) with postoperative radiotherapy (RT) have been uncertain. RADICALS-HD compared adding no ("None"), 6-months ("Short"), or 24-mo ("Long") ADT to study efficacy in the long term.
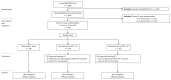
Methods: Participants with prostate cancer were indicated for postoperative RT and agreed randomisation between all durations. ADT was allocated for 0, 6, or 24 mo. The primary outcome measure (OM) was metastasis-free survival (MFS). The secondary OMs included freedom from distant metastasis, overall survival, and initiation of nonprotocol ADT. Sample size was determined by two-way comparisons. Analyses followed standard time-to-event approaches and intention-to-treat principles.
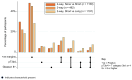
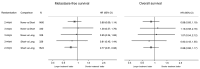
Key findings and limitations: Between 2007 and 2015, 492 participants were randomised one of three groups: 166 None, 164 Short, and 162 Long. The median age at randomisation was 66 yr; Gleason scores at surgery were as follows: <7 = 64 (13%), 3+4 = 229 (47%), 4+3 = 127 (26%), and 8+ = 72 (15%); T3b was 112 (23%); and T4 was 5 (1%). The median follow-up was 9.0 yr and, with MFS events reported for 89 participants (32 None, 31 Short, and 26 Long), there was no evidence of difference in MFS overall (logrank p = 0.98), and, for Long versus None, hazard ratio = 0.948 (95% confidence interval 0.54-1.68). After 10 yr, 80% None, 77% Short, and 81% Long patients were alive without metastatic disease. The three-way randomisation was not powered to conventional levels for assessment, yet provides a fair comparison.
Conclusions and clinical implications: Long-term outcomes after radical prostatectomy are usually favourable. In those indicated for postoperative RT and considered suitable for no, short-term, or long-term ADT, there was no evidence of improvement with addition of ADT. Future research should focus on patients at a higher risk of metastases in whom improvements are required more urgently.
Keywords: Androgen deprivation therapy; Clinical trials; Duration; Hormone therapy; Multiarm trial; Prostate cancer; Radical prostatectomy; Radiotherapy; Randomised controlled trial.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Matthew R. Sydes certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chris C. Parker reports consulting fees from AAA, ITM Radiopharma, Myovant, and Clarity Pharmaceuticals to his institution, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen and Bayer to his institution. Noel W. Clarke reports honoraria for lectures, advisory boards, and symposia from AstraZeneca, Janssen, Bayer, and Pfizer; reports support for travel to and attendance at a European meeting from Bayer; reports participation in the independent data monitoring committee for the Probio trial (Karolinska), and the trial steering committee for the Capi 28 trial (AstraZeneca) and STAMPEDE trial (MRC); and is the Joint National Clinical Lead for the National Prostate Cancer Audit. Adrian D. Cook reports research grants for the STAMPEDE trial from Janssen, Astellas, Novartis, Sanofi, and Clovis. Peter M. Petersen reports participation in a data safety monitoring board or advisory board for AAA Nordic, MSD, and Pfizer Denmark. Charles N. Catton reports support for the present manuscript from the Canadian Cancer Trials Group, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bayer Corp, Knight Therapeutics, and AbbVie. William R. Cross reports honoraria for lectures, advisory boards, and symposia from Bayer, Janssen, AAA Novartis, Astellas and support for travel to and attendance of conferences from Bayer, Janssen and Ipsen. Claire Amos reports research grants for STAMPEDE-1 and STAMPEDE-2 trials from Janssen, Astellas, Novartis, Sanofi, and Clovis Oncology. Fred Saad serves as a consultant and has received honoraria and the institution has received funding for clinical trials from: Abbvie, AstraZeneca, astellas, Bayer, Janssen, Merck, Pfizer and Novartis. Ian Sayers received sponsorship for conference attendance from IPSEN. Lorna Bower reports that the previous Institute of Cancer role was funded in part by a Biomedical Research Centre grant, which was paid to the institution; the role was not connected to the RADICALS trial. Fred Saad served as a consultant and has received honoraria and his institution has received funding for clinical trials from: Abbvie, AstraZeneca, Astellas, Bayer, Janssen, Merck, Pfizer, Novartis. Mahesh K.B. Parmar reports research grants for the STAMPEDE trial from Janssen, Astellas, Novartis, Sanofi, and Clovis. Matthew R. Sydes reports research grants and biomarker testing costs, all to their institution and all active in the past 36 mo, but on research outside of this research, from Astellas, Clovis Oncology, Janssen, Novartis, and Sanofi-Aventis; reports consulting fees from Eli Lilly; speaker fees at a clinical trial statistics training meeting for clinicians (no discussion of particular drugs) from Lilly Oncology, Janssen, and Eisai; and is an independent member of many independent data monitoring committees, but all for academic sponsors and unpaid. The remaining authors have nothing to disclose.
Figures

References
-
- Parker CC, Clarke NW, Catton C, et al. RADICALS-HD: reflections before the results are known. Clin Oncol (R Coll Radiol) 2022;34:593–7. - PubMed
-
- Parker C, Clarke N, Cook A, et al. LBA9—Duration of androgen deprivation therapy (ADT) with post-operative radiotherapy (RT) for prostate cancer: first results of the RADICALS-HD trial (ISRCTN40814031) Ann Oncol. 2022;33:S808–69.
-
- Cook AD, Parker C, Parmar M, Sydes M. RADICALS trial statistical analysis plan. 2022.
-
- Parker CC, Clarke NW, Cook AD, et al. Adding 6 months of androgen deprivation therapy to postoperative radiotherapy for prostate cancer: a comparison of short-course versus no androgen deprivation therapy in the RADICALS-HD randomised controlled trial. Lancet. 2024;403:2405–15. doi: 10.1016/S0140-6736(24)00548-8. - DOI - PMC - PubMed
-
- Parker CC, Kynaston H, Cook AD, et al. Duration of androgen deprivation therapy with postoperative radiotherapy for prostate cancer: a comparison of long-course versus short-course androgen deprivation therapy in the RADICALS-HD randomised trial. Lancet. 2024;403:2416–25. doi: 10.1016/S0140-6736(24)00549-X. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

