Pleiotropy in FOXC1-attributable phenotypes involves altered ciliation and cilia-dependent signaling
- PMID: 39217245
- PMCID: PMC11365983
- DOI: 10.1038/s41598-024-71159-y
Pleiotropy in FOXC1-attributable phenotypes involves altered ciliation and cilia-dependent signaling
Abstract
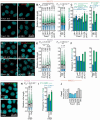
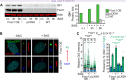
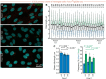
Alterations to cilia are responsible for a wide range of severe disease; however, understanding of the transcriptional control of ciliogenesis remains incomplete. In this study we investigated whether altered cilia-mediated signaling contributes to the pleiotropic phenotypes caused by the Forkhead transcription factor FOXC1. Here, we show that patients with FOXC1-attributable Axenfeld-Rieger Syndrome (ARS) have a prevalence of ciliopathy-associated phenotypes comparable to syndromic ciliopathies. We demonstrate that altering the level of Foxc1 protein, via shRNA mediated inhibition, CRISPR/Cas9 mutagenesis and overexpression, modifies cilia length in vitro. These structural changes were associated with substantially perturbed cilia-dependent signaling [Hedgehog (Hh) and PDGFRα], and altered ciliary compartmentalization of the Hh pathway transcription factor, Gli2. Consistent with these data, in primary cultures of murine embryonic meninges, cilia length was significantly reduced in heterozygous and homozygous Foxc1 mutants compared to controls. Meningeal expression of the core Hh signaling components Gli1, Gli3 and Sufu was dysregulated, with comparable dysregulation of Pdgfrα signaling evident from significantly altered Pdgfrα and phosphorylated Pdgfrα expression. On the basis of these clinical and experimental findings, we propose a model that altered cilia-mediated signaling contributes to some FOXC1-induced phenotypes.
Keywords: Axenfeld–Rieger syndrome; FOXC1; Hedgehog; PDGFRα signaling; Primary cilia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

