Digital consults in heart failure care: a randomized controlled trial
- PMID: 39217271
- PMCID: PMC11485254
- DOI: 10.1038/s41591-024-03238-6
Digital consults in heart failure care: a randomized controlled trial
Erratum in
-
Author Correction: Digital consults in heart failure care: a randomized controlled trial.Nat Med. 2024 Oct;30(10):3027. doi: 10.1038/s41591-024-03322-x. Nat Med. 2024. PMID: 39367252 Free PMC article. No abstract available.
Abstract
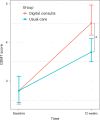
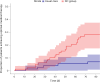
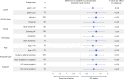
Guideline-directed medical therapy (GDMT) has clear benefits on morbidity and mortality in patients with heart failure; however, GDMT use remains low. In the multicenter, open-label, investigator-initiated ADMINISTER trial, patients (n = 150) diagnosed with heart failure and reduced ejection fraction (HFrEF) were randomized (1:1) to receive usual care or a strategy using digital consults (DCs). DCs contained (1) digital data sharing from patient to clinician (pharmacotherapy use, home-measured vital signs and Kansas City Cardiomyopathy Questionnaires); (2) patient education via a text-based e-learning; and (3) guideline recommendations to all treating clinicians. All remotely gathered information was processed into a digital summary that was available to clinicians in the electronic health record before every consult. All patient interactions were standardly conducted remotely. The primary endpoint was change in GDMT score over 12 weeks (ΔGDMT); this GDMT score directly incorporated all non-conditional class 1 indications for HFrEF therapy with equal weights. The ADMINISTER trial met its primary outcome of achieving a higher GDMT in the DC group after a follow-up of 12 weeks (ΔGDMT score in the DC group: median 1.19, interquartile range (0.25, 2.3) arbitrary units versus 0.08 (0.00, 1.00) in usual care; P < 0.001). To our knowledge, this is the first multicenter randomized controlled trial that proves a DC strategy is effective to achieve GDMT optimization. ClinicalTrials.gov registration: NCT05413447 .
© 2024. The Author(s).
Conflict of interest statement
M.L.H. is supported by the Dutch Heart Foundation (Dr. E. Dekker Senior Clinical Scientist Grant 2020T058) and CVON (2020B008 RECONNEXT). M.L.H. received an investigator-initiated research grant from Vifor Pharma; an educational grant from Boehringer Ingelheim and Novartis; and speaker/consultancy fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Daiichi Sankyo, Quin and Vifor Pharma. W.E.M.K. received a speaking fee from Novartis. F.W.A. received grant funding from the European Union Horizon scheme (AI4HF 101080430 and DataTools4Heart 101057849). M.J.S. received an independent research grant from AstraZeneca to the research institute. The other authors declare no competing interests.
Figures

References
-
- Savarese, G. et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res.118, 3272–3287 (2023). - PubMed
-
- McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J.42, 3599–3726 (2021). - PubMed
-
- Fatima, K., Butler, J. & Fonarow, G. C. Residual risk in heart failure and the need for simultaneous implementation and innovation. Eur. J. Heart Fail.25, 1477–1480 (2023). - PubMed
-
- Tromp, J. et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail.10, 73–84 (2022). - PubMed
-
- Fauvel, C. et al. Differences between heart failure specialists and non‐specialists regarding heart failure drug implementation and up‐titration. Eur. J. Heart Fail.25, 1884–1886 (2023). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

