Cellular senescence and SASP in tumor progression and therapeutic opportunities
- PMID: 39217404
- PMCID: PMC11365203
- DOI: 10.1186/s12943-024-02096-7
Cellular senescence and SASP in tumor progression and therapeutic opportunities
Abstract
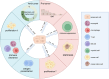
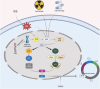
Cellular senescence (CS), a permanent and irreversible arrest of the cell cycle and proliferation leading to the degeneration of cellular structure and function, has been implicated in various key physiological and pathological processes, particularly in cancer. Initially, CS was recognized as a barrier to tumorigenesis, serving as an intrinsic defense mechanism to protect cells from malignant transformation. However, increasing evidence suggests that senescent cells can promote tumor progression to overt malignancy, primarily through a set of factors known as senescence-associated secretory phenotypes (SASPs), including chemokines, growth factors, cytokines, and stromal metalloproteinases. These factors significantly reshape the tumor microenvironment (TME), enabling tumors to evade immune destruction. Interestingly, some studies have also suggested that SASPs may impede tumor development by enhancing immunosurveillance. These opposing roles highlight the complexity and heterogeneity of CS and SASPs in diverse cancers. Consequently, there has been growing interest in pharmacological interventions targeting CS or SASPs in cancer therapy, such as senolytics and senomorphics, to either promote the clearance of senescent cells or mitigate the harmful effects of SASPs. In this review, we will interpret the concept of CS, delve into the role of SASPs in reshaping the TME, and summarize recent advances in anti-tumor strategies targeting CS or SASPs.
Keywords: Cellular senescence; SASP; Therapy; Tumor; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

