Prediction of incident atrial fibrillation using deep learning, clinical models, and polygenic scores
- PMID: 39217446
- PMCID: PMC11631091
- DOI: 10.1093/eurheartj/ehae595
Prediction of incident atrial fibrillation using deep learning, clinical models, and polygenic scores
Abstract
Background and aims: Deep learning applied to electrocardiograms (ECG-AI) is an emerging approach for predicting atrial fibrillation or flutter (AF). This study introduces an ECG-AI model developed and tested at a tertiary cardiac centre, comparing its performance with clinical models and AF polygenic score (PGS).
Methods: Electrocardiograms in sinus rhythm from the Montreal Heart Institute were analysed, excluding those from patients with pre-existing AF. The primary outcome was incident AF at 5 years. An ECG-AI model was developed by splitting patients into non-overlapping data sets: 70% for training, 10% for validation, and 20% for testing. The performance of ECG-AI, clinical models, and PGS was assessed in the test data set. The ECG-AI model was externally validated in the Medical Information Mart for Intensive Care-IV (MIMIC-IV) hospital data set.
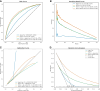
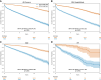
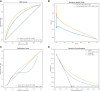
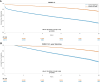
Results: A total of 669 782 ECGs from 145 323 patients were included. Mean age was 61 ± 15 years, and 58% were male. The primary outcome was observed in 15% of patients, and the ECG-AI model showed an area under the receiver operating characteristic (AUC-ROC) curve of .78. In time-to-event analysis including the first ECG, ECG-AI inference of high risk identified 26% of the population with a 4.3-fold increased risk of incident AF (95% confidence interval: 4.02-4.57). In a subgroup analysis of 2301 patients, ECG-AI outperformed CHARGE-AF (AUC-ROC = .62) and PGS (AUC-ROC = .59). Adding PGS and CHARGE-AF to ECG-AI improved goodness of fit (likelihood ratio test P < .001), with minimal changes to the AUC-ROC (.76-.77). In the external validation cohort (mean age 59 ± 18 years, 47% male, median follow-up 1.1 year), ECG-AI model performance remained consistent (AUC-ROC = .77).
Conclusions: ECG-AI provides an accurate tool to predict new-onset AF in a tertiary cardiac centre, surpassing clinical and PGS.
Keywords: Atrial fibrillation; Deep learning; Electrocardiogram; Polygenic scores.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





References
-
- Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation 2016;133:484–92. 10.1161/CIRCULATIONAHA.115.018614 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

