Zibotentan in Microvascular Angina: A Randomized, Placebo-Controlled, Crossover Trial
- PMID: 39217504
- PMCID: PMC11573082
- DOI: 10.1161/CIRCULATIONAHA.124.069901
Zibotentan in Microvascular Angina: A Randomized, Placebo-Controlled, Crossover Trial
Abstract
Background: Microvascular angina is associated with dysregulation of the endothelin system and impairments in myocardial blood flow, exercise capacity, and health-related quality of life. The G allele of the noncoding single nucleotide polymorphism RS9349379 enhances expression of the endothelin-1 gene (EDN1) in human vascular cells, potentially increasing circulating concentrations of Endothelin-1 (ET-1). Whether zibotentan, an oral ET-A receptor selective antagonist, is efficacious and safe for the treatment of microvascular angina is unknown.
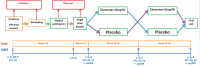
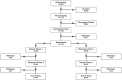
Methods: Patients with microvascular angina were enrolled in this double-blind, placebo-controlled, sequential crossover trial of zibotentan (10 mg daily for 12 weeks). The trial population was enriched to ensure a G allele frequency of 50% for the RS9349379 single nucleotide polymorphism. Participants and investigators were blinded to genotype. The primary outcome was treadmill exercise duration (seconds) using the Bruce protocol. The primary analysis estimated the mean within-participant difference in exercise duration after treatment with zibotentan versus placebo.
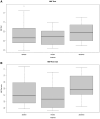
Results: A total of 118 participants (mean±SD; years of age 63.5 [9.2]; 71 [60.2%] females; 25 [21.2%] with diabetes) were randomized. Among 103 participants with complete data, the mean exercise duration with zibotentan treatment compared with placebo was not different (between-treatment difference, -4.26 seconds [95% CI, -19.60 to 11.06] P=0.5871). Secondary outcomes showed no improvement with zibotentan. Zibotentan reduced blood pressure and increased plasma concentrations of ET-1. Adverse events were more common with zibotentan (60.2%) compared with placebo (14.4%; P<0.001).
Conclusions: Among patients with microvascular angina, short-term treatment with a relatively high dose (10 mg daily) of zibotentan was not beneficial. Target-related adverse effects were common.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04097314.
Keywords: angina with no obstructive coronary arteries; endothelin receptor antagonist; exercise test; genetics; microvascular angina; pharmacotherapy.
Conflict of interest statement
Dr Berry is employed by the University of Glasgow, which holds consultancy and research agreements with Abbott Vascular, AstraZeneca, Auxilius Pharma, Boehringer Ingelheim, Corflow, Coroventis, GlaxoSmithKline, HeartFlow, Menarini, Novartis, Siemens Healthcare, Somalogic, Xylocor and Valo Health. Dr Spyridopoulos receives research grants from Astra Zeneca, Cambridge, UK, and Kancera, Solna, Sweden. Dr Ford is a consultant/speaker/honorarium from Abbott Vascular, Boston Scientific, Boehringer Ingelheim, Biotronik, Bio-Excel, and Novartis. Dr Al-Lamee serves on advisory boards of Janssen Pharmaceuticals, Abbott, and Philips, and has received speaker honoraria from Abbott, Philips, Medtronic, Servier, Omniprex, and Menarini. These companies had no role in the design or conduct of the study or in the data collection or interpretation. Dr Davenport holds research grants from AstraZeneca and is a member of scientific advisory boards of Janssen, ENB Therapeutics, and Pharmazz. Drs Berry, Ford, and Davenport are named on a pending patent for the use of zibotentan for microvascular angina. The University of Glasgow holds the patent. None of the other authors have any relevant disclosures.
Figures
References
-
- Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369 - PubMed
-
- Samuels BA, Shah SM, Widmer RJ, Kobayashi Y, Miner SES, Taqueti VR, Jeremias A, Albadri A, Blair JA, Kearney KE, et al. ; Microvascular Network (MVN). Comprehensive management of ANOCA, part 1-Definition, patient population, and diagnosis: JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;82:1245–1263. doi: 10.1016/j.jacc.2023.06.043 - PubMed
-
- Smilowitz NR, Prasad M, Widmer RJ, Toleva O, Quesada O, Sutton NR, Lerman A, Reynolds HR, Kesarwani M, Savage MP, et al. ; Microvascular Network (MVN). Comprehensive management of ANOCA, part 2-Program development, treatment, and research initiatives: JACC State-of-the-Art Review. J Am Coll Cardiol. 2023;82:1264–1279. doi: 10.1016/j.jacc.2023.06.044 - PubMed
-
- Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6:S188–S191. doi: 10.1097/00004872-198812040-00056 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

