Strain surveillance during chemotherapy to improve cardiovascular outcomes: the SUCCOUR-MRI trial
- PMID: 39217601
- PMCID: PMC11542702
- DOI: 10.1093/eurheartj/ehae574
Strain surveillance during chemotherapy to improve cardiovascular outcomes: the SUCCOUR-MRI trial
Abstract
Background and aims: The detection of cancer therapy-related cardiac dysfunction (CTRCD) by reduction of left ventricular ejection fraction (LVEF) during chemotherapy usually triggers the initiation of cardioprotective therapy. This study addressed whether the same approach should be applied to patients with worsening of global longitudinal strain (GLS) without attaining thresholds of LVEF.
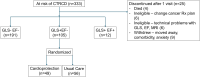
Methods: Strain surveillance during chemotherapy for improving cardiovascular outcomes (SUCCOUR-MRI) was a prospective multicentre randomized controlled trial involving 14 sites. Of 355 patients receiving anthracyclines with normal baseline LVEF, 333 patients (age 59 ± 13 years, 79% women) with at least one other CTRCD risk factor, able to undergo magnetic resonance imaging (MRI), GLS, and three-dimensional echocardiography were tracked over 12 months. A total of 105 patients (age 59 ± 13 years, 75% women, 69% breast cancer) developing GLS-CTRCD (>12% relative reduction of GLS without a change in LVEF) were randomized to cardioprotection with neurohormonal antagonists vs. usual care. The primary endpoint was 12-month change in MRI-LVEF; the secondary endpoint was MRI-LVEF-defined CTRCD.
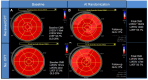
Results: During follow-up, two patients died, and two developed heart failure. Most patients were randomized at 3 months (62%). Median doses of angiotensin inhibition/blockade and beta-blockade were 75% and 50% of respective targets; 21 (43%) had side-effects attributed to cardioprotection. Due to a smaller LVEF change from baseline with cardioprotection than usual care (-2.5 ± 5.4% vs. -5.6 ± 5.9%, P = .009), follow-up LVEF was higher after cardioprotection (59 ± 5% vs. 55 ± 6%, P < .0001). After adjustment for baseline LVEF, the mean (95% confidence interval) difference in the change in LVEF between the two groups was -3.6% (-1.8% to -5.5%, P < .001). After cardioprotection, 1/49 patients developed 12-month LVEF-CTRCD, compared to 6/56 in usual care (P = .075). Global longitudinal strain improved at 3 months post-randomization in the cardioprotection group, with little change with usual care.
Conclusions: In patients with isolated GLS reduction after anthracyclines, cardioprotection is associated with better preservation of 12-month MRI-LVEF compared with usual care.
Keywords: Cancer therapy-related cardiac dysfunction; Global longitudinal strain; Left ventricular ejection fraction.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

