Aldehydes alter TGF-β signaling and induce obesity and cancer
- PMID: 39217614
- PMCID: PMC11560041
- DOI: 10.1016/j.celrep.2024.114676
Aldehydes alter TGF-β signaling and induce obesity and cancer
Abstract
Obesity and fatty liver diseases-metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH)-affect over one-third of the global population and are exacerbated in individuals with reduced functional aldehyde dehydrogenase 2 (ALDH2), observed in approximately 560 million people. Current treatment to prevent disease progression to cancer remains inadequate, requiring innovative approaches. We observe that Aldh2-/- and Aldh2-/-Sptbn1+/- mice develop phenotypes of human metabolic syndrome (MetS) and MASH with accumulation of endogenous aldehydes such as 4-hydroxynonenal (4-HNE). Mechanistic studies demonstrate aberrant transforming growth factor β (TGF-β) signaling through 4-HNE modification of the SMAD3 adaptor SPTBN1 (β2-spectrin) to pro-fibrotic and pro-oncogenic phenotypes, which is restored to normal SMAD3 signaling by targeting SPTBN1 with small interfering RNA (siRNA). Significantly, therapeutic inhibition of SPTBN1 blocks MASH and fibrosis in a human model and, additionally, improves glucose handling in Aldh2-/- and Aldh2-/-Sptbn1+/- mice. This study identifies SPTBN1 as a critical regulator of the functional phenotype of toxic aldehyde-induced MASH and a potential therapeutic target.
Keywords: ALDH2; CP: Cancer; CP: Metabolism; HCC; MASH; SMAD3; SPTBN1; TGF-β; cancer; liver disease; metabolic syndrome; reactive aldehydes.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




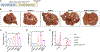

References
-
- Dingler FA, Wang M, Mu A, Millington CL, Oberbeck N, Watcham S, Pontel LB, Kamimae-Lanning AN, Langevin F, Nadler C, et al. (2020). Two Aldehyde Clearance Systems Are Essential to Prevent Lethal Formaldehyde Accumulation in Mice and Humans. Mol. Cell. 80, 996–1012.e9. 10.1016/j.molcel.2020.10.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

