Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination
- PMID: 39217987
- PMCID: PMC11464205
- DOI: 10.1016/j.immuni.2024.08.004
Meningeal lymphatic function promotes oligodendrocyte survival and brain myelination
Abstract
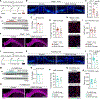
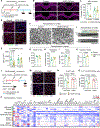
The precise neurophysiological changes prompted by meningeal lymphatic dysfunction remain unclear. Here, we showed that inducing meningeal lymphatic vessel ablation in adult mice led to gene expression changes in glial cells, followed by reductions in mature oligodendrocyte numbers and specific lipid species in the brain. These phenomena were accompanied by altered meningeal adaptive immunity and brain myeloid cell activation. During brain remyelination, meningeal lymphatic dysfunction provoked a state of immunosuppression that contributed to delayed spontaneous oligodendrocyte replenishment and axonal loss. The deficiencies in mature oligodendrocytes and neuroinflammation due to impaired meningeal lymphatic function were solely recapitulated in immunocompetent mice. Patients diagnosed with multiple sclerosis presented reduced vascular endothelial growth factor C in the cerebrospinal fluid, particularly shortly after clinical relapses, possibly indicative of poor meningeal lymphatic function. These data demonstrate that meningeal lymphatics regulate oligodendrocyte function and brain myelination, which might have implications for human demyelinating diseases.
Keywords: brain myelin; demyelination; immune cells; meningeal lymphatic vessels; multiple sclerosis; neuroinflammation; oligodendrocytes; oxidative stress; remyelination; vascular endothelial growth factor C.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.D.M. is listed as an inventor in patents and patent applications concerning modulating lymphatic vessels in neurological diseases (University of Virginia Licensing & Ventures Group and PureTech Ventures LLC). S.R.I. has received honoraria/research support from UCB, Immunovant, MedImmun, Roche, Janssen, Cerebral therapeutics, ADC therapeutics, Brain, CSL Behring, and ONO Pharma, receives licensed royalties on patent application WO/2010/046716 entitled “Neurological Autoimmune Disorders,” and has filed two other patents entitled “Diagnostic method and therapy” (WO2019211633 and US-2021-0071249-A1; PCT application WO202189788A1) and “Biomarkers” (PCT/GB2022/050614 and WO202189788A1).
Figures



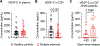
Comment in
-
Oligodendrocytes go with the flow: Meningeal lymphatics promote myelin integrity.Immunity. 2024 Oct 8;57(10):2255-2257. doi: 10.1016/j.immuni.2024.09.006. Immunity. 2024. PMID: 39383839
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

