Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney and mortality outcomes
- PMID: 39218030
- PMCID: PMC11645272
- DOI: 10.1038/s41591-024-03264-4
Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney and mortality outcomes
Erratum in
-
Author Correction: Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney and mortality outcomes.Nat Med. 2024 Dec;30(12):3778. doi: 10.1038/s41591-024-03372-1. Nat Med. 2024. PMID: 39468366 Free PMC article. No abstract available.
Abstract
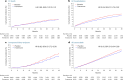
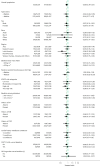
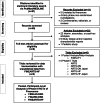
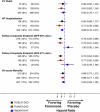
Cardiovascular-kidney-metabolic syndrome is an emerging entity that connects cardiovascular diseases, chronic kidney disease and diabetes. The non-steroidal mineralocorticoid receptor antagonist finerenone has been studied in three prospective randomized clinical trials of patients with cardiovascular-kidney-metabolic syndrome: FIDELIO-DKD, FIGARO-DKD and FINEARTS-HF. In light of the strong epidemiological overlap and shared mechanistic drivers of clinical outcomes across cardiovascular-kidney-metabolic syndrome, we summarize the efficacy and safety of finerenone on cardiovascular, kidney and mortality outcomes in this pre-specified participant-level pooled analysis. The three trials included 18,991 participants (mean age 67 ± 10 years; 35% women). During 2.9 years of median follow-up, the primary outcome of cardiovascular death occurred in 421 (4.4%) participants assigned to finerenone and 471 (5.0%) participants assigned to placebo (hazard ratio (HR): 0.89; 95% confidence interval (CI): 0.78-1.01; P = 0.076). Death from any cause occurred in 1,042 (11.0%) participants in the finerenone arm and in 1,136 (12.0%) participants in the placebo arm (HR: 0.91; 95% CI: 0.84-0.99; P = 0.027). Finerenone further reduced the risk of hospitalization from heart failure (HR: 0.83; 95% CI: 0.75-0.92; P < 0.001) and the composite kidney outcome (HR: 0.80; 95% CI: 0.72-0.90; P < 0.001). While in this pooled analysis the reduction in cardiovascular death was not statistically significant, finerenone reduced the risks for deaths of any cause, cardiovascular events and kidney outcomes. PROSPERO identifier: CRD42024570467 .
© 2024. The Author(s).
Conflict of interest statement
Competing interests: M.V. has received research grant support, served on advisory boards or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Bristol Myers Squibb, Boehringer Ingelheim, Chiesi, Cytokinetics, Fresenius Medical Care, Idorsia Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Milestone Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi and Tricog Health and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech and Impulse Dynamics. G.F. has received lecture fees from Bayer, Boehringer Ingelheim, Servier and Novartis; trial committee membership fees from Bayer, Boehringer Ingelheim, Servier, Impulse Dynamics, Vifor and Medtronic; consulting fees from Cardior and Novo Nordisk; and research grants from the European Union. B.L.C. has received personal consulting fees from Alnylam, Bristol Myers Squibb, Cardior, Cardurion, Corvia, CVRx, Eli Lilly, Intellia and Rocket and has served on a data and safety monitoring board (DSMB) for Novo Nordisk. A.S.S. has received institutional research grants (to Brigham and Women’s Hospital) from Abbott, Alnylam, AstraZeneca, Bayer, Novartis and Pfizer as well as personal consulting fees from Abbott, Alnylam, AstraZeneca, Bayer, Biofourmis, Boston Scientific, Medpace, Medtronic, Merck, Novartis, Parexel, Porter Health, Regeneron, River2Renal, Roche, Veristat, Verily and Zydus. P.S.J. reports speaker fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications and Sun Pharmaceuticals; advisory board fees from AstraZeneca, Boehringer Ingelheim and Novartis; and research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices and Roche Diagnostics. P.S.J.’s employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer AG, Novartis and Novo Nordisk. Director of the Global Clinical Trial Partners Ltd. A.D. has no financial conflicts to report. M.B. is a full-time employee of Bayer AG. P.K. is a full-time employee of Bayer AG. He is also a co-inventor of finerenone and holds US and European patents relating to finerenone (US8436180B2 and EP2132206B1). P.S. is an employee of Bayer AG. J.L.-F. is a full-time employee of Bayer plc, Research & Development, Pharmaceuticals. P.V. is an employee of Bayer AG. C.S.P.L. has received research support from NovoNordisk and Roche Diagnostics; has received consulting fees from Alleviant Medical, Allysta Pharma, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Biopeutics, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, CardioRenal, CPC Clinical Research, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development, Medscape/WebMD Global, Merck, Novartis, Novo Nordisk, Prosciento, Quidel Corporation, Radcliffe Group, Recardio ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and is a co-founder and non-executive director of Us2.ai. M.S. has served on advisory boards for and has received consultancy fees and honoraria from Novartis, Abbott, Merck, Merck Sharp & Dohme, Vifor, AstraZeneca, Cardurion, Novonordisk, Bayer and Boehringer Ingelheim. S.J.S. has received research grants from the National Institutes of Health (NIH) (U54 HL160273, X01 HL169712, R01 HL140731 and R01 HL149423), the American Heart Association (AHA) (24SFRNPCN1291224), AstraZeneca, Corvia and Pfizer and consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Eli Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya and Ultromics. A.A.V.ʼs employer received consultancy fees and/or research support from Adrenomed, Anacardio, AstraZeneca, Bayer AG, Bristol Myers Squibb, Boehringer Ingelheim, Corteria, Eli Lilly, Merck, Moderna, Novartis, Novo Nordisk, Roche Diagnostics and SalubrisBio. F.Z. reports personal fees from 89Bio, Abbott, Acceleron, Applied Therapeutics, Bayer, Betagenon, Boehringer, Bristol Myers Squibb, CVRx, Cambrian, Cardior, Cereno Pharmaceutical, Cellprothera, CEVA, Inventiva, KBP, Merck, NovoNordisk, Owkin, Otsuka, Roche Diagnostics, Northsea and USa2; having stock options at G3Pharmaceutical and equities at Cereno, Cardiorenal, Eshmoun Clinical Research; and being the founder of Cardiovascular Clinical Trialists. P.R. reports grants and payment of honoraria for lectures, educational events and steering group participation from AstraZeneca, Bayer and Novo Nordisk (all to the Steno Diabetes Center Copenhagen); payment of honoraria for lectures and participation in advisory boards from Boehringer Ingelheim, Sanofi, Gilead and Astellas (all to the Steno Diabetes Center Copenhagen); and receipt of study drugs for free for investigator-initiated studies from Bayer, Novo Nordisk and Lexicon. L.M.R. has no financial conflicts to report. S.D.A. reports grants from Vifor and Abbott; consulting fees from CVRx, Astra Zeneca, Bioventrix, Repairon, Novo Nordisk, Brahms, Novartis, Actimed Therapeutics, Faraday Pharmaceuticals, Cytokinetics, HeartKinetics, GlaxoSmithKline, Vectorious, Scirent, Sensible Medical, Edwards, Relaxera, Repairon, Regeneron Pharmaceuticals and Cordio; steering or advisory committee work for Vifor, Bayer AG, Boehringer Ingelheim, Medtronic, Abbott, Impulse Dynamics, Cardior, V-Wave, Pfizer, Cardiac Dimensions and Occlutech; and being named as a co-inventor of two patent applications regarding MR-proANP (DE 102007010834 and DE 102007022367), but he does not benefit personally from the related issued patents. B.P. has served as a consultant for Bayer, Astra Zeneca, Bristol Meyers Squibb, Boehringer Ingelheim, Lexicon, Anacardia and G3 Pharmaceuticvals. He has served as a consultant and received stock options or stocks from Sea Star Medical, Vifor, scPharmaceuticals, SQinnovations, KBP Biosciences, Sarfez, Cereno Scientific, Prointel and Brainstorm Medical. He holds a US patent (9931412: Site specific delivery of eplerenone to the myocardium) and has a US patent pending (63/045,783: Histone modulating agents for the prevention and treatment of organ damage). R.A. reports support from Bayer; royalties or licenses from UpToDate; consulting fees from Boehringer Ingelheim, Novartis, Akebia, Intercept Pharma and Alnylam; support for meetings from Boehringer Ingelheim, Novartis, Akebia and Vertex; and participation on DSMBs or advisory boards for Vertex, Eloxx and Chinook. J.J.V.M. reports payments through the University of Glasgow from work on clinical trials, consulting and grants from Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GlaxoSmithKline and Novartis; personal consultancy fees from Alynylam Pharmaceuticals, Amgen, AnaCardio, AstraZeneca, Bayer, Berlin Cures, Bristol Myers Squibb, Cardurion, Cytokinetics, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, River 2 Renal Corp., the British Heart Foundation, the NIH National Heart, Lung, and Blood Institute (NHLBI), Boehringer Ingelheim, SQ Innovations and Catalyze Group; personal lecture fees from Abbott, Alkem Metabolics, AstraZeneca, Blue Ocean Scientific Solutions, Boehringer Ingelheim, Canadian Medical and Surgical Knowledge, Emcure Pharmaceuticals, Eris Lifesciences, European Academy of CME, Hikma Pharmaceuticals, Imagica Health, Intas Pharmaceuticals, J.B. Chemicals & Pharmaceuticals, Lupin Pharmaceuticals, Medscape/Heart.Org., ProAdWise Communications, Radcliffe Cardiology, Sun Pharmaceuticals, The Corpus, Translation Research Group and Translational Medicine Academy; and DSMB for WIRB-Copernicus Group Clinical. He is also a director of Global Clinical Trial Partners, Ltd. S.D.S. has received research grants from Alexion, Alnylam, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Boston Scientific, Cytokinetics, Edgewise, Eidos, Gossamer, GlaxoSmithKline, Ionis, Eli Lilly, MyoKardia, NIH/NHLBI, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos and US2.AI and has consulted for Abbott, Action, Akros, Alexion, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, GlaxoSmithKline, Eli Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros and Valo.
Figures



Comment in
-
Finerenone improves outcomes in HFmrEF and HFpEF.Nat Rev Cardiol. 2024 Nov;21(11):739. doi: 10.1038/s41569-024-01087-x. Nat Rev Cardiol. 2024. PMID: 39300251 No abstract available.
References
-
- Ostrominski, J. W. et al. Cardiovascular-kidney-metabolic overlap in heart failure with mildly reduced or preserved ejection fraction: a trial-level analysis. J. Am. Coll. Cardiol.84, 223–228 (2024). - PubMed
-
- Ndumele, C. E. et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation148, 1606–1635 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

