GLT-1 downregulation in hippocampal astrocytes induced by type 2 diabetes contributes to postoperative cognitive dysfunction in adult mice
- PMID: 39218798
- PMCID: PMC11366448
- DOI: 10.1111/cns.70024
GLT-1 downregulation in hippocampal astrocytes induced by type 2 diabetes contributes to postoperative cognitive dysfunction in adult mice
Abstract
Aims: Type 2 diabetes mellitus (T2DM) is related to an increased risk of postoperative cognitive dysfunction (POCD), which may be caused by neuronal hyperexcitability. Astrocyte glutamate transporter 1 (GLT-1) plays a crucial role in regulating neuron excitability. We investigated if T2DM would magnify the increased neuronal excitability induced by anesthesia/surgery (A/S) and lead to POCD in young adult mice, and if so, determined whether these effects were associated with GLT-1 expression.
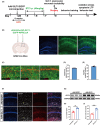
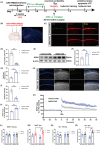
Methods: T2DM model was induced by high fat diet (HFD) and injecting STZ. Then, we evaluated the spatial learning and memory of T2DM mice after A/S with the novel object recognition test (NORT) and object location test (OLT). Western blotting and immunofluorescence were used to analyze the expression levels of GLT-1 and neuronal excitability. Oxidative stress reaction and neuronal apoptosis were detected with SOD2 expression, MMP level, and Tunel staining. Hippocampal functional synaptic plasticity was assessed with long-term potentiation (LTP). In the intervention study, we overexpressed hippocampal astrocyte GLT-1 in GFAP-Cre mice. Besides, AAV-Camkllα-hM4Di-mCherry was injected to inhibit neuronal hyperexcitability in CA1 region.
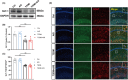
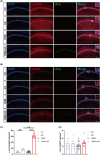
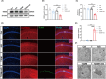
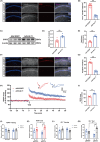
Results: Our study found T2DM but not A/S reduced GLT-1 expression in hippocampal astrocytes. Interestingly, GLT-1 deficiency alone couldn't lead to cognitive decline, but the downregulation of GLT-1 in T2DM mice obviously enhanced increased hippocampal glutamatergic neuron excitability induced by A/S. The hyperexcitability caused neuronal apoptosis and cognitive impairment. Overexpression of GLT-1 rescued postoperative cognitive dysfunction, glutamatergic neuron hyperexcitability, oxidative stress reaction, and apoptosis in hippocampus. Moreover, chemogenetic inhibition of hippocampal glutamatergic neurons reduced oxidative stress and apoptosis and alleviated postoperative cognitive dysfunction.
Conclusions: These findings suggest that the adult mice with type 2 diabetes are at an increased risk of developing POCD, perhaps due to the downregulation of GLT-1 in hippocampal astrocytes, which enhances increased glutamatergic neuron excitability induced by A/S and leads to oxidative stress reaction, and neuronal apoptosis.
Keywords: GLT‐1; apoptosis; neuronal hyperexcitability; postoperative cognitive dysfunction.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18‐30. - PubMed
-
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, ISPOCD Group . Long‐term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548‐555. - PubMed
-
- Edipoglu IS, Celik F. The associations between cognitive dysfunction, stress biomarkers, and administered anesthesia type in Total knee arthroplasties: prospective, randomized trial. Pain Physician. 2019;22(5):495‐507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

