Suppression of the JAK/STAT pathway inhibits neuroinflammation in the line 61-PFF mouse model of Parkinson's disease
- PMID: 39218899
- PMCID: PMC11368013
- DOI: 10.1186/s12974-024-03210-8
Suppression of the JAK/STAT pathway inhibits neuroinflammation in the line 61-PFF mouse model of Parkinson's disease
Abstract
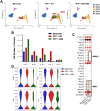
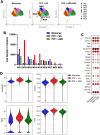
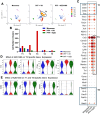
Parkinson's disease (PD) is characterized by neuroinflammation, progressive loss of dopaminergic neurons, and accumulation of α-synuclein (α-Syn) into insoluble aggregates called Lewy pathology. The Line 61 α-Syn mouse is an established preclinical model of PD; Thy-1 is used to promote human α-Syn expression, and features of sporadic PD develop at 9-18 months of age. To accelerate the PD phenotypes, we injected sonicated human α-Syn preformed fibrils (PFFs) into the striatum, which produced phospho-Syn (p-α-Syn) inclusions in the substantia nigra pars compacta and significantly increased MHC Class II-positive immune cells. Additionally, there was enhanced infiltration and activation of innate and adaptive immune cells in the midbrain. We then used this new model, Line 61-PFF, to investigate the effect of inhibiting the JAK/STAT signaling pathway, which is critical for regulation of innate and adaptive immune responses. After administration of the JAK1/2 inhibitor AZD1480, immunofluorescence staining showed a significant decrease in p-α-Syn inclusions and MHC Class II expression. Flow cytometry showed reduced infiltration of CD4+ T-cells, CD8+ T-cells, CD19+ B-cells, dendritic cells, macrophages, and endogenous microglia into the midbrain. Importantly, single-cell RNA-Sequencing analysis of CD45+ cells from the midbrain identified 9 microglia clusters, 5 monocyte/macrophage (MM) clusters, and 5 T-cell (T) clusters, in which potentially pathogenic MM4 and T3 clusters were associated with neuroinflammatory responses in Line 61-PFF mice. AZD1480 treatment reduced cell numbers and cluster-specific expression of the antigen-presentation genes H2-Eb1, H2-Aa, H2-Ab1, and Cd74 in the MM4 cluster and proinflammatory genes such as Tnf, Il1b, C1qa, and C1qc in the T3 cluster. Together, these results indicate that inhibiting the JAK/STAT pathway suppresses the activation and infiltration of innate and adaptive cells, reducing neuroinflammation in the Line 61-PFF mouse model.
Keywords: AZD1480; JAK/STAT; Neuroinflammation; Parkinson’s disease; scRNA-Seq; α-Synuclein.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Suppression of the JAK/STAT Pathway Inhibits Neuroinflammation in the Line 61-PFF Mouse Model of Parkinson's Disease.Res Sq [Preprint]. 2024 May 7:rs.3.rs-4307273. doi: 10.21203/rs.3.rs-4307273/v1. Res Sq. 2024. Update in: J Neuroinflammation. 2024 Sep 1;21(1):216. doi: 10.1186/s12974-024-03210-8. PMID: 38766241 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

