Reduced-dose chemotherapy and blinatumomab as induction treatment for newly diagnosed Ph-negative B-cell precursor acute lymphoblastic leukemia: a phase 2 trial
- PMID: 39218935
- PMCID: PMC11367979
- DOI: 10.1186/s13045-024-01597-8
Reduced-dose chemotherapy and blinatumomab as induction treatment for newly diagnosed Ph-negative B-cell precursor acute lymphoblastic leukemia: a phase 2 trial
Abstract
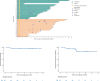
Blinatumomab has emerged as a promising component of first-line therapy for acute B-cell precursor lymphoblastic leukemia (BCP-ALL), bolstering treatment efficacy. To mitigate CD19 selection pressure and reduce the incidence of blinatumomab-associated toxicities, pre-treatment chemotherapy is recommended before administering blinatumomab. From September 2022 to December 2023, we conducted a single-arm, multicenter, phase 2 trial (NCT05557110) in newly diagnosed Philadelphia chromosome-negative BCP-ALL (Ph-negative BCP-ALL) patients. Participants received induction treatment with reduced-dose chemotherapy (RDC), comprising idarubicin, vindesine, and dexamethasone over 7 days, followed by 2 weeks of blinatumomab. Those failing to achieve composite complete remission (CRc) received an additional 2 weeks of blinatumomab. The primary endpoint was the CRc rate post initial induction treatment. Of the 35 enrolled patients, 33 (94%) achieved CRc after 2 weeks of blinatumomab, with 30 (86%) achieving measurable residual disease (MRD) negativity. Two patients extended blinatumomab to 4 weeks. With either 2 or 4 weeks of blinatumomab treatment, all patients achieved CR (35/35) and 89% (31/35) were MRD negativity. The median time to CR was 22 days. Immune effector cell-associated neurotoxicity syndrome was limited (14%, all grade 1). Non-hematological adverse events of grade 3 or higher included pneumonia (17%), sepsis (6%), and cytokine release syndrome (9%). With a median follow-up of 11.5 months, estimated 1-year overall survival and 1-year progression-free survival rates were 97.1% and 82.2%, respectively. These findings affirm that RDC followed by blinatumomab is an effective and well-tolerated induction regimen for newly diagnosed Ph-negative BCP-ALL, supporting a shift towards less intensive and more targeted therapeutic approaches. Trial registration: https://www.clinicaltrials.Gov . Identifier NCT05557110.
Keywords: B-cell precursor acute lymphoblastic leukemia; Blinatumomab; Induction treatment; Philadelphia chromosome-negative; Reduced-dose chemotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–801. 10.1002/cncr.20668 - DOI - PubMed
-
- Cabannes-Hamy A, Brissot E, Leguay T, Huguet F, Chevallier P, Hunault M, et al. High tumor burden before blinatumomab has a negative impact on the outcome of adult patients with B-cell precursor acute lymphoblastic leukemia. A real-world study by the GRAALL. Haematologica. 2022;107(9):2072–80. 10.3324/haematol.2021.280078 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical