Bone marrow stromal and anterior cruciate ligament remnant cell co-culture-derived extracellular vesicles promote cell activity in both cell types
- PMID: 39219013
- PMCID: PMC11366498
- DOI: 10.1111/jcmm.70049
Bone marrow stromal and anterior cruciate ligament remnant cell co-culture-derived extracellular vesicles promote cell activity in both cell types
Abstract
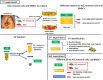
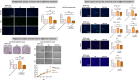
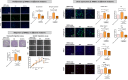
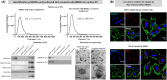
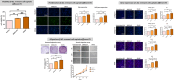
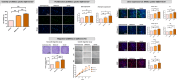
The significance of anterior cruciate ligament (ACL) remnants during reconstruction remains unclear. Co-culturing ACL remnant cells and bone marrow stromal cells (BMSCs) may reduce apoptosis and enhance hamstring tendon activity. This study investigated whether extracellular vesicles (EVs), which facilitate cell-cell interactions, act as the active components, improving graft maturation in this co-culture. The effects of EVs on cell viability, proliferation, migration and gene expression in the rabbit ACL remnant cells and BMSCs were assessed using control (BMSC-only culture), co-culture (ACL remnant cells and BMSCs, CM) and co-culture without EVs (CM ∆ EVs) media. EVs were isolated from control (BMSC-EV) and co-culture (CM-EV) media and characterized. CM significantly enhanced the proliferation, migration and expression of transforming growth factor (TGF-β)-, vascular endothelial growth factor (VEGF)-, collagen synthesis- and tenogenesis-related genes. However, CM-induced effects were reversed by the CM ∆ EVs treatment. CM-EV treatment exhibited higher potential to enhance proliferation, migration and gene expression in the ACL remnant cells and BMSCs than BMSC-EV and non-EV treatments. In conclusion, EVs, secreted under the coexistence of ACL remnant cells and BMSCs, primarily increase the cell viability, proliferation, migration and gene expression of collagen synthesis-, TGF-β-, VEGF- and tenogenesis-related genes in both cell types.
Keywords: anterior cruciate ligament; bone marrow stromal cells; extracellular vesicle; tenogenesis.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
Cheng‐Chang Lu and all authors, or any member of his or her immediate family has no funding or commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted article. I agree and confirm that this statement is true.
Figures
Similar articles
-
Anterior cruciate ligament remnant preservation attenuates apoptosis and enhances the regeneration of hamstring tendon graft.Bone Joint Res. 2023 Jan;12(1):9-21. doi: 10.1302/2046-3758.121.BJR-2021-0434.R2. Bone Joint Res. 2023. PMID: 36617435 Free PMC article.
-
Extracorporeal shock wave promotes activation of anterior cruciate ligament remnant cells and their paracrine regulation of bone marrow stromal cells' proliferation, migration, collagen synthesis, and differentiation.Bone Joint Res. 2020 Aug 11;9(8):458-468. doi: 10.1302/2046-3758.98.BJR-2019-0365.R1. eCollection 2020 Aug. Bone Joint Res. 2020. PMID: 32832074 Free PMC article.
-
Mechanical Loading Improves Tendon-Bone Healing in a Rabbit Anterior Cruciate Ligament Reconstruction Model by Promoting Proliferation and Matrix Formation of Mesenchymal Stem Cells and Tendon Cells.Cell Physiol Biochem. 2017;41(3):875-889. doi: 10.1159/000460005. Epub 2017 Feb 16. Cell Physiol Biochem. 2017. PMID: 28214894
-
Single extracellular vesicle research: From cell population to a single cell.Biochem Biophys Res Commun. 2024 Nov 19;734:150439. doi: 10.1016/j.bbrc.2024.150439. Epub 2024 Jul 23. Biochem Biophys Res Commun. 2024. PMID: 39083971 Review.
-
The Role of Extracellular Vesicles in β-Cell Function and Viability: A Scoping Review.Front Endocrinol (Lausanne). 2020 Jun 11;11:375. doi: 10.3389/fendo.2020.00375. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32595604 Free PMC article.
References
-
- Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Art Ther. 2002;18(5):502‐509. - PubMed
-
- Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population‐based study. Sci Med Sport. 2009;12(6):622‐627. - PubMed
-
- Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21‐year population‐based study. Am J Sports Med. 2016;44(6):1502‐1507. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

