Utility of cerebrovascular imaging biomarkers to detect cerebral amyloidosis
- PMID: 39219209
- PMCID: PMC11485066
- DOI: 10.1002/alz.14207
Utility of cerebrovascular imaging biomarkers to detect cerebral amyloidosis
Abstract
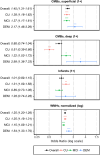
Introduction: The relationship between cerebrovascular disease (CVD) and amyloid beta (Aβ) in Alzheimer's disease (AD) is understudied. We hypothesized that magnetic resonance imaging (MRI)-based CVD biomarkers-including cerebral microbleeds (CMBs), lacunar infarction, and white matter hyperintensities (WMHs)-would correlate with Aβ positivity on positron emission tomography (Aβ-PET).
Methods: We cross-sectionally analyzed data from the Alzheimer's Disease Neuroimaging Initiative (ADNI, N = 1352). Logistic regression was used to calculate odds ratios (ORs), with Aβ-PET positivity as the standard-of-truth.
Results: Following adjustment, WMHs (OR = 1.25) and superficial CMBs (OR = 1.45) remained positively associated with Aβ-PET positivity (p < 0.001). Deep CMBs and lacunes exhibited a varied relationship with Aβ-PET in cognitive subgroups. The combined diagnostic model, which included CVD biomarkers and other accessible measures, significantly predicted Aβ-PET (pseudo-R2 = 0.41).
Discussion: The study highlights the translational value of CVD biomarkers in diagnosing AD, and underscores the need for more research on their inclusion in diagnostic criteria.
Clinicaltrials: gov: ADNI-2 (NCT01231971), ADNI-3 (NCT02854033).
Highlights: Cerebrovascular biomarkers linked to amyloid beta (Aβ) in Alzheimer's disease (AD). White matter hyperintensities and cerebral microbleeds reliably predict Aβ-PET positivity. Relationships with Aβ-PET vary by cognitive stage. Novel accessible model predicts Aβ-PET status. Study supports multimodal diagnostic approaches.
Keywords: ADNI; Alzheimer's disease; amyloid beta; cerebrovascular disease; magnetic resonance imaging; positron emission tomography; small vessel disease.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
M. Howe: None to disclose. M. Caruso: None to disclose. M. Manoochehri: None to disclose. Z. Kunicki: None to disclose. S. Emrani: None to disclose. J. Rudolph: None to disclose. E. Huey: None to disclose. S. Salloway: Dr. Salloway has provided consultation to Biogen, Eisai, Avid, Lilly, Genentech, and Roche. H. Oh: None to disclose. Butler Hospital has received research grants from Biogen, Eisai, Avid, Roche, Genentech, Janssen, and Lilly. Author disclosures are available in the supporting information.
Figures
Update of
-
Utility of cerebrovascular imaging biomarkers to detect cerebral amyloidosis.medRxiv [Preprint]. 2024 May 28:2024.05.28.24308056. doi: 10.1101/2024.05.28.24308056. medRxiv. 2024. Update in: Alzheimers Dement. 2024 Oct;20(10):7220-7231. doi: 10.1002/alz.14207. PMID: 38853879 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Associated data
Grants and funding
- Lumosity; Lundbeck; Merck & Co., Inc.
- U01 AG079850/AG/NIA NIH HHS/United States
- CAPMC/ CIHR/Canada
- GE Healthcare
- Janssen Alzheimer Immunotherapy Research & Development, LLC.
- Transition Therapeutics
- EB/NIBIB NIH HHS/United States
- F. Hoffmann-La Roche Ltd
- Elan Pharmaceuticals, Inc.
- 2P01AG051449-06/AG/NIA NIH HHS/United States
- Eisai Inc.
- W81XWH-12-2-0012/Department of Defense
- EuroImmun
- Biogen
- Johnson & Johnson Pharmaceutical Research & Development LLC.
- R25 MH101076/MH/NIMH NIH HHS/United States
- R01 AG068990/AG/NIA NIH HHS/United States
- Alzheimer's Drug Discovery Foundation
- Servier
- Bristol-Myers Squibb Company
- U01 AG024904/AG/NIA NIH HHS/United States
- Piramal Imaging
- Takeda Pharmaceutical Company
- ALZ/Alzheimer's Association/United States
- Genentech, Inc.
- Araclon Biotech
- U01 AG024904/NH/NIH HHS/United States
- Novartis Pharmaceuticals Corporation
- Meso Scale Diagnostics, LLC.
- CereSpir, Inc.
- R01AG068990/AG/NIA NIH HHS/United States
- BioClinica, Inc.
- 2R25MH101076-06A1/MH/NIMH NIH HHS/United States
- R01 MH120794/MH/NIMH NIH HHS/United States
- Cogstate
- P01 AG051449/AG/NIA NIH HHS/United States
- Eli Lilly and Company
- R01 AG062268/AG/NIA NIH HHS/United States
- IXICO Ltd.
- NeuroRx Research
- Fujirebio
- Neurotrack Technologies
LinkOut - more resources
Full Text Sources
Medical

