Molecular mechanisms of PI3K isoform dependence in embryonic growth
- PMID: 39219229
- PMCID: PMC11576644
- DOI: 10.4274/jtgga.galenos.2024.2024-6-7
Molecular mechanisms of PI3K isoform dependence in embryonic growth
Abstract
Objective: The phosphoinositide 3-kinase (PI3K) pathway is an important signaling mechanism for cell proliferation and metabolism. Mutations that activate PIK3CA may make cells p110α dependent, but when phosphatase tensin homolog (PTEN) is lost, the p110β isoform of PI3Ks becomes more important. However, the exact mechanism underlying the prevalence of p110s remains unclear. In this study, our aim was to elucidate the processes behind PI3K isoform dependency in a cellular model of embryonic development.
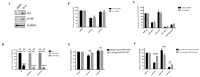
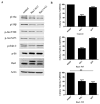
Material and methods: In order to understand PI3K isoform prevalence, mouse embryonic fibroblasts (MEFs) were used and p110β, PTEN and Rac1 activity was modulated using retroviral plasmids. Expression levels and cellular growth were assessed by performing immunoblots and crystal violet assays.
Results: The levels of PTEN had only a partial effect on the prevalence of PI3K isoforms in MEFs. The dependency on p110α diminished when PTEN was depleted. Of note, when PTEN expression was repressed, there was no full transition in dependency from one PI3K isoform to the other. Interestingly, the viability of PTEN-depleted MEFs became less dependent on p110α and more dependent on p110β when p110β was overexpressed. Nevertheless, the overexpression of p110β in conjunction with PTEN knock-downs did not result in a complete shift of isoforms in PI3Ks. Finally, we investigated Rac1 activation with a mutant allele and determined a more potent increase in p110β prominence in MEFs.
Conclusion: These findings suggest that multiple cellular parameters, including PTEN status, PI3K isoform levels, and Rac1 activity, combine to influence PI3K isoform prevalence, rather than a single determinant.
Keywords: PI3K isoform prominence; PTEN; Rac1; mouse embryonic fibroblasts.
Conflict of interest statement
Conflict of Interest: No conflict of interest is declared by the authors.
Figures

Similar articles
-
PI3K isoform dependence of PTEN-deficient tumors can be altered by the genetic context.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6395-400. doi: 10.1073/pnas.1323004111. Epub 2014 Apr 15. Proc Natl Acad Sci U S A. 2014. PMID: 24737887 Free PMC article.
-
The phosphatidylinositol 3-kinase (PI3K) isoform dependence of tumor formation is determined by the genetic mode of PI3K pathway activation rather than by tissue type.J Virol. 2014 Sep;88(18):10673-9. doi: 10.1128/JVI.01409-14. Epub 2014 Jul 2. J Virol. 2014. PMID: 24991009 Free PMC article.
-
Both p110α and p110β isoforms of PI3K can modulate the impact of loss-of-function of the PTEN tumour suppressor.Biochem J. 2012 Feb 15;442(1):151-9. doi: 10.1042/BJ20111741. Biochem J. 2012. PMID: 22150431 Free PMC article.
-
Class I Phosphoinositide 3-Kinase PIK3CA/p110α and PIK3CB/p110β Isoforms in Endometrial Cancer.Int J Mol Sci. 2018 Dec 7;19(12):3931. doi: 10.3390/ijms19123931. Int J Mol Sci. 2018. PMID: 30544563 Free PMC article. Review.
-
p110α and p110β isoforms of PI3K signaling: are they two sides of the same coin?FEBS Lett. 2016 Sep;590(18):3071-82. doi: 10.1002/1873-3468.12377. Epub 2016 Sep 12. FEBS Lett. 2016. PMID: 27552098 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
