Outcomes of transcatheter aortic valve implantation for native aortic valve regurgitation
- PMID: 39219361
- PMCID: PMC11363660
- DOI: 10.4244/EIJ-D-24-00339
Outcomes of transcatheter aortic valve implantation for native aortic valve regurgitation
Abstract
Background: Large datasets of transcatheter aortic valve implantation (TAVI) for pure aortic valve regurgitation (PAVR) are scarce.
Aims: We aimed to report procedural safety and long-term clinical events (CE) in a contemporary cohort of PAVR patients treated with new-generation devices (NGD).
Methods: Patients with grade III/IV PAVR enrolled in the FRANCE-TAVI Registry were selected. The primary safety endpoint was technical success (TS) according to Valve Academic Research Consortium 3 criteria. The co-primary endpoint was defined as a composite of mortality, heart failure hospitalisation and valve reintervention at last follow-up.
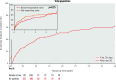
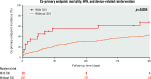
Results: From 2015 to 2021, 227 individuals (64.3% males, median age 81.0 [interquartile range {IQR} 73.5-85.0] years, with EuroSCORE II 6.0% [IQR 4.0-10.9]) from 41 centres underwent TAVI with NGD, using either self-expanding (55.1%) or balloon-expandable valves (44.9%; p=0.50). TS was 85.5%, with a non-significant trend towards increased TS in high-volume activity centres. A second valve implantation (SVI) was needed in 8.8% of patients, independent of valve type (p=0.82). Device size was ≥29 mm in 73.0% of patients, post-procedure grade ≥III residual aortic regurgitation was rare (1.2%), and the permanent pacemaker implantation (PPI) rate was 36.0%. At 30 days, the incidences of mortality and reintervention were 8.4% and 3.5%, respectively. The co-primary endpoint reached 41.6% (IQR 34.4-49.6) at 1 year, increased up to 61.8% (IQR 52.4-71.2) at 4 years, and was independently predicted by TS, with a hazard ratio of 0.45 (95% confidence interval: 0.27-0.76); p=0.003.
Conclusions: TAVI with NGD in PAVR patients is efficient and reasonably safe. Preventing the need for an SVI embodies the major technical challenge. Larger implanted valves may have limited this complication, outweighing the increased risk of PPI. Despite successful TAVI, PAVR patients experience frequent CE at long-term follow-up.
Conflict of interest statement
L. Leroux has served as a proctor for Medtronic. T. Lefèvre has served as a proctor for Edwards Lifesciences and Abbott. D. Tchétché has received honoraria or consultation fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. B. Iung has received consulting fees from Boehringer Ingelheim; and has received speaker fees from Edwards Lifesciences. H. Le Breton has received speaker fees from Edwards Lifesciences and Medtronic. H. Eltchaninoff has served as a proctor for and received lecture fees from Edwards Lifesciences. J.-P. Collet has received research grants from Bristol-Myers Squibb and Medtronic; and lecture fees from Bristol-Myers Squibb, Bayer, Daichii Sankyo, AstraZeneca, and Medtronic. J.-F. Obadia received consulting or speaker fees from Abbott, Delacroix-Chevalier, and Medtronic. E. Teiger has served as a proctor for Medtronic. C. Saint-Etienne has received honoraria from Abbott and Biotronik. The other authors have no conflicts of interest to declare relevant to the contents of this paper.
Figures

References
-
- Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897–902. - PubMed
-
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. - PubMed
-
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. - PMC - PubMed
-
- Yoon SH, Schmidt T, Bleiziffer S, Schofer N, Fiorina C, Munoz-Garcia AJ, Yzeiraj E, Amat-Santos IJ, Tchetche D, Jung C, Fujita B, Mangieri A, Deutsch MA, Ubben T, Deuschl F, Kuwata S, De Biase, Williams T, Dhoble A, Kim WK, Ferrari E, Barbanti M, Vollema EM, Miceli A, Giannini C, Attizzani GF, Kong WKF, Gutierrez-Ibanes E, Jimenez Diaz, Wijeysundera HC, Kaneko H, Chakravarty T, Makar M, Sievert H, Hengstenberg C, Prendergast BD, Vincent F, Abdel-Wahab M, Nombela-Franco L, Silaschi M, Tarantini G, Butter C, Ensminger SM, Hildick-Smith D, Petronio AS, Yin WH, De Marco, Testa L, Van Mieghem, Whisenant BK, Kuck KH, Colombo A, Kar S, Moris C, Delgado V, Maisano F, Nietlispach F, Mack MJ, Schofer J, Schaefer U, Bax JJ, Frerker C, Latib A, Makkar RR. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J Am Coll Cardiol. 2017;70:2752–63. - PubMed
-
- De Backer, Pilgrim T, Simonato M, Mackensen GB, Fiorina C, Veulemanns V, Cerillo A, Schofer J, Amabile N, Achkouty G, Schäfer U, Deutsch MA, Sinning JM, Rahman MS, Sawaya FJ, Hildick-Smith D, Hernandez JM, Kim WK, Lefevre T, Seiffert M, Bleiziffer S, Petronio AS, Van Mieghem, Taramasso M, Søndergaard L, Windecker S, Latib A, Dvir D. Usefulness of Transcatheter Aortic Valve Implantation for Treatment of Pure Native Aortic Valve Regurgitation. Am J Cardiol. 2018;122:1028–35. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

