Topical Anti-Inflammatory Treatments for Eczema: A Cochrane Systematic Review and Network Meta-Analysis
- PMID: 39219446
- PMCID: PMC11629051
- DOI: 10.1111/cea.14556
Topical Anti-Inflammatory Treatments for Eczema: A Cochrane Systematic Review and Network Meta-Analysis
Abstract
Objective: Eczema is the most burdensome skin condition worldwide and topical anti-inflammatory treatments are commonly used to control symptoms. The relative effectiveness and safety of different topical anti-inflammatory treatments is uncertain.
Design: Network meta-analysis performed within a Cochrane systematic review to compare and statistically rank efficacy and safety of topical anti-inflammatory eczema treatments.
Data sources: Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase and trial registries to June 2023.
Eligibility criteria for selected trials: Included trials were within-participant or between-participant randomised controlled trials. Participants had eczema that was not clinically infected and was not contact dermatitis, seborrheic eczema or hand eczema. Interventions were topical anti-inflammatory treatments but not complementary treatments, antibiotics alone, wet wraps, phototherapy or systemic treatments. Comparators were no treatment/vehicle or another topical anti-inflammatory.
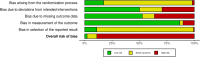
Results: We identified 291 trials (45,846 participants), mainly in high-income countries. Most were industry-funded with median 3 weeks treatment duration. Risk of bias assessed using the Cochrane Risk of Bias 2.0 tool was high in 89% of trials, mainly due to risk of selective reporting. Network meta-analysis of binary outcomes ranked potent and/or very potent topical steroids, tacrolimus 0.1% and ruxolitinib 1.5% among the most effective treatments for improving patient-reported symptoms (40 trials, all low confidence) and clinician-reported signs (32 trials, all moderate confidence). For investigator global assessment, the Janus kinas inhibitors ruxolitinib 1.5%, delgocitinib 0.5% or 0.25%, very potent/potent topical steroids and tacrolimus 0.1% were ranked as most effective (140 trials, all moderate confidence). Continuous outcome data were mixed. Local application site reactions were most common with tacrolimus 0.1% (moderate confidence) and crisaborole 2% (high confidence) and least common with topical steroids (moderate confidence). Skin thinning was not increased with short-term use of any topical steroid potency (low confidence) but skin thinning was reported in 6/2044 (0.3%) participants treated with longer-term (6-60 months) topical steroids.
Conclusion: Potent topical steroids, Janus kinase inhibitors and tacrolimus 0.1% were consistently ranked as among the most effective topical anti-inflammatory treatments for eczema.
Keywords: Janus kinase inhibitor; calcineurin inhibitor; eczema; network meta‐analysis; systematic review; topical steroid.
© 2024 The Author(s). Clinical & Experimental Allergy published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare the following interests. S.J.L.: NIHR grants, published evidence syntheses and knowledge mobilisation work on the topic and an editorial regarding an included trial. R.P.: Commissioning Editor, The Cochrane Collaboration. E.A.: employed by Cochrane as an Evidence Synthesis Methodology Editor within the Cochrane Methods Support Unit. D.K.C.: author on AAAAI/ACAAI Atopic Dermatitis topical treatments systematic review and guideline. M.F.: payments from Maruho, Otsuka Pharmaceutical, Torii Pharmaceutical; H.C.W.: investigator on an included trial. Suzie Cro: NIHR advanced fellowship. A.M.D.: author of Canadian Dermatology Today; dermatologist at Women's College Hospital; Vice Chair of Scientific and Medical Advisory Committee and research grants from the National Eczema Association. Consultant for Canadian Association for Drugs and Technology in Health; Editor of Cochrane Skin. R.J.B.: fees for editorial work from Wiley and the British Society for Allergy and Clinical Immunology, and for expert witness work in relation to allergy. All other authors declare no conflict of interest.
Figures


References
-
- Langan S. M., Mulick A. R., Rutter C. E., et al., “Trends in Eczema Prevalence in Children and Adolescents: A Global Asthma Network Phase I Study,” Clinical and Experimental Allergy 53, no. 3 (2023): 337–352.
-
- Hay R. J., Johns N. E., Williams H. C., et al., “The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions,” Journal of Investigative Dermatology 134, no. 6 (2014): 1527–1534. - PubMed
-
- Paolino A., Alexander H., Broderick C., and Flohr C., “Non‐biologic Systemic Treatments for Atopic Dermatitis: Current State of the Art and Future Directions,” Clinical and Experimental Allergy 53 (2023): 495–510. - PubMed
-
- David E., Ungar B., Renert‐Yuval Y., Facheris P., Del Duca E., and Guttman‐Yassky E., “The Evolving Landscape of Biologic Therapies for Atopic Dermatitis: Present and Future Perspective,” Clinical and Experimental Allergy 53 (2023): 156–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

