Pufferfish gasdermin Ea is a significant player in the defense against bacterial pathogens
- PMID: 39219679
- PMCID: PMC11358365
- DOI: 10.1007/s42995-024-00237-x
Pufferfish gasdermin Ea is a significant player in the defense against bacterial pathogens
Abstract
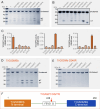
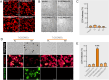
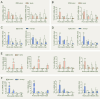
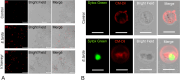
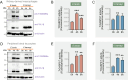
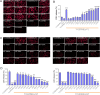
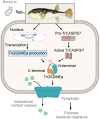
Gasdermins (GSDMs) are proteins cleaved by caspase (CASP) to trigger pyroptosis. In teleosts, pyroptosis is mediated by gasdermin E (GSDME). The Pufferfish, Takifugu rubripes, possesses two GSDME orthologs: named TrGSDMEa and TrGSDMEb. TrGSDMEa is cleaved by CASP3/7 to liberate the N-terminal (NT) domain that can trigger pyroptosis in mammalian cells. However, the biological function of TrGSDMEa in pufferfish is unknown, and TrGSDMEb is poorly studied. We found that TrGSDMEb was cleaved by CASP1/3/6/7/8, but the resulting NT domain, despite its similarity to TrGSDMEa-NT domain in sequence and structure, failed to induce pyroptosis. TrGSDMEa and TrGSDMEb exhibited similar expression patterns in pufferfish under normal physiological conditions but were up- and downregulated, respectively, in expression during Vibrio harveyi and Edwardsiella tarda infection. Bacterial infection induced the activation of TrGSDMEa and CASP3/7 in pufferfish cells, resulting in pyroptosis accompanied with IL-1β production and maturation. Inhibition of TrGSDMEa-mediated pyroptosis via TrCASP3/7 reduced the death of pufferfish cells and augmented bacterial dissemination in fish tissues. Structure-oriented mutagenesis identified 16 conserved residues in teleost GSDMEa that were required for the pore formation or auto-inhibition of GSDMEa. This study illustrates the role of GSDMEa-mediated pyroptosis in teleost defense against bacterial pathogens and provides new insights into the structure-based function of vertebrate GSDME.
Supplementary information: The online version contains supplementary material available at 10.1007/s42995-024-00237-x.
Keywords: Bacterial infection; Caspase; GSDME; Immune defense; Takifugu rubripes.
© The Author(s) 2024.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures

References
-
- Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S et al (2002) Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 297:1301–1310 - PubMed
-
- Booty LM, Bryant CE (2022) Gasdermin D and beyond - gasdermin-mediated pyroptosis in bacterial infections. J Mol Biol 434:167409 - PubMed
-
- Brenner S, Elgar G, Sandford R, Macrae A, Venkatesh B, Aparicio S (1993) Characterization of the pufferfish (Fugu) genome as a compact model vertebrate genome. Nature 366:265–268 - PubMed
-
- Broz P, Pelegrin P, Shao F (2020) The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol 20:143–157 - PubMed
-
- Chen H, Wu X, Gu Z, Chen S, Zhou X, Zhang Y, Liu Q, Wang Z, Yang D (2021) Zebrafish gasdermin E cleavage-engaged pyroptosis by inflammatory and apoptotic caspases. Dev Comp Immunol 124:104203 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

