Delivery of a STING Agonist Using Lipid Nanoparticles Inhibits Pancreatic Cancer Growth
- PMID: 39220196
- PMCID: PMC11365503
- DOI: 10.2147/IJN.S462213
Delivery of a STING Agonist Using Lipid Nanoparticles Inhibits Pancreatic Cancer Growth
Abstract
Introduction: The tumor microenvironment (TME) of pancreatic cancer is highly immunosuppressive and characterized by a large number of cancer-associated fibroblasts, myeloid-derived suppressor cells, and regulatory T cells. Stimulator of interferon genes (STING) is an endoplasmic reticulum receptor that plays a critical role in immunity. STING agonists have demonstrated the ability to inflame the TME, reduce tumor burden, and confer anti-tumor activity in mouse models. 2'3' cyclic guanosine monophosphate adenosine monophosphate (2'3'-cGAMP) is a high-affinity endogenous ligand of STING. However, delivering cGAMP to antigen-presenting cells and tumor cells within the cytosol remains challenging due to membrane impermeability and poor stability.
Methods: In this study, we encapsulated 2'3'-cGAMP in a lipid nanoparticle (cGAMP-LNP) designed for efficient cellular delivery. We assessed the properties of the nanoparticles using a series of in-vitro studies designed to evaluate their cellular uptake, cytosolic release, and minimal cytotoxicity. Furthermore, we examined the nanoparticle's anti-tumor effect in a syngeneic mouse model of pancreatic cancer.
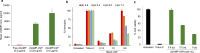
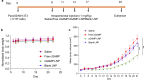
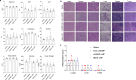
Results: The lipid platform significantly increased the cellular uptake of 2'3'-cGAMP. cGAMP-LNP exhibited promising antitumor activity in the syngeneic mouse model of pancreatic cancer.
Discussion: The LNP platform shows promise for delivering exogenous 2'3'-cGAMP or its derivatives in cancer therapy.
Keywords: 2’3’-cGAMP; cold tumor; cytosolic delivery; innate immunotherapy; lipid nanoparticle.
© 2024 Shaji et al.
Conflict of interest statement
Kun Cheng reports a patent application for the lipids used in this article. The authors report no other conflicts of interest in this work.
Figures


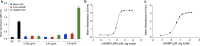
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

