Are natural estrogens used in contraception at lower risk of venous thromboembolism than synthetic ones? A systematic literature review and meta-analysis
- PMID: 39220361
- PMCID: PMC11362054
- DOI: 10.3389/fendo.2024.1428597
Are natural estrogens used in contraception at lower risk of venous thromboembolism than synthetic ones? A systematic literature review and meta-analysis
Abstract
Background: Venous thromboembolism (VTE) poses a significant global health challenge, notably exacerbated by the use of combined oral contraceptives (COCs). Evidence mainly focuses on the type of progestogen used in COCs to establish the increased risk of VTE with less data assessed on the type of estrogen used. This meta-analysis aims to assess the risk of VTE associated with COCs containing synthetic estrogens like ethinylestradiol (EE) versus natural estrogens like estradiol (E2).
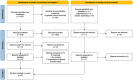
Methods: A systematic review and meta-analysis was conducted following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Literature searches were performed in December 2023 in MEDLINE and EMBASE to identify clinical studies comparing the VTE risk between COCs containing synthetic versus natural estrogens. Studies were selected through rigorous screening, and data extraction followed standardized protocols, with statistical analyses employing a random effects model.
Results: The search yielded five relevant studies, involving over 560,000 women/time, demonstrating a significant 33% reduction in VTE risk among users of natural estrogen-based COCs compared to synthetic estrogen-based COCs (OR 0.67, 95% CI 0.51-0.87). Stratification analyses using adjusted hazard ratios (HR) of the main observationnal studies showed a 49% reduced VTE risk of E2-based pills compared to EE in association with levonorgestrel.
Discussion and conclusion: Despite the longstanding use of EE-based COCs, emerging evidence supports a lower thrombotic risk associated with natural estrogens. This meta-analysis substantiates the lower VTE risk associated with natural estrogen-based COCs compared to synthetic alternatives, advocating for a re-evaluation of contraceptive guidelines to prioritize patient safety and reduce thrombotic risks.
Keywords: combined oral contraceptive; estradiol; ethinylestradiol; meta-analysis; venous thromboembolism.
Copyright © 2024 Douxfils, Raskin, Didembourg, Donis, Dogné, Morimont and Beaudart.
Conflict of interest statement
Authors JD, MD, ND, and LM were employed by company Qualiblood sa. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
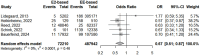
References
-
- European Medicines Agency . Assessment report for combined hormonal contraceptives containing medicinal products — Procedure number: EMEA/H/A-31/1356. Pharmacovigilance Risk Assessment Committee; (2014). Available at: https://www.ema.europa.eu/en/documents/referral/combined-hormonal-contra....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

