Prenatal cell-free DNA testing of women with pregnancy-associated cancer: a retrospective cross-sectional study
- PMID: 39220433
- PMCID: PMC11363838
- DOI: 10.1016/j.lanepe.2024.101024
Prenatal cell-free DNA testing of women with pregnancy-associated cancer: a retrospective cross-sectional study
Abstract
Background: Incidentally, the non-invasive prenatal test (NIPT) shows chromosomal aberrations suspicious of a maternal malignancy, especially after genome-wide testing. The aim of this study is to determine how many cases of cancer in pregnancy are diagnosed or missed with NIPT and whether in retrospect subtle changes in NIPT results could have detected cancer.
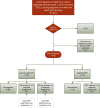
Methods: We identified Dutch patients diagnosed in 2017-2021 with pregnancy-associated cancer from the International Network on Cancer, Infertility and Pregnancy (INCIP) Registry, who underwent NIPT in the Dutch NIPT implementation study (TRIDENT-2). We retrospectively assessed how many of these women showed a malignancy suspicious-NIPT, their tumour types and -stages, and the time interval between NIPT and cancer diagnosis.
Findings: Of 143 women with pregnancy-associated cancer, we included 65 patients that underwent an NIPT. Fifty-four women had a solid tumour and 11 a haematological malignancy. Sixteen (24.6%) NIPTs were malignancy suspicious (15 genome-wide, one targeted). All 10 haematological cancer patients with genome-wide NIPT had a malignancy suspicious-NIPT, irrespective of the disease stage. Only five patients with a solid tumour had a genome-wide malignancy suspicious-NIPT (4/5 advanced cancer stage III or IV). The mean time between date of NIPT and cancer diagnosis was significantly shorter after a malignancy suspicious-NIPT compared to a non-suspicious-NIPT, respectively 49.9 days (± SD 31.8) and 100.7 days (± SD 74.9), p = 0.001.
Interpretation: All genome-wide NIPT in women with pregnancy-associated haematological malignancies were malignancy suspicious. Women with a solid tumour showed a malignancy suspicious-NIPT in only a minority of cases, mainly the advanced stages.
Funding: None.
Keywords: Noninvasive prenatal test; Pregnancy-associated cancer.
© 2024 The Authors.
Conflict of interest statement
VTH participated in the advisory board of AstraZeneca, E Lilly and Novartis; received grants for the institution outside the present work, from AstraZeneca, E Lilly, Gilead, Novartis and Pfizer. All other authors declare no competing interests.
Figures


References
-
- Andersson T.M., Johansson A.L.V., Hsieh C.C., Cnattingius S., Lambe M. Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol. 2009;114(3):568–572. - PubMed
-
- Pavlidis N.A. Coexistence of pregnancy and malignancy. Oncol. 2002;7(4):279–287. - PubMed
-
- International Network on Cancer Infertility and Pregnancy Cancer in pregnancy n.d. https://www.cancerinpregnancy.org Available from:
LinkOut - more resources
Full Text Sources

