The effectiveness of nirsevimab in reducing the burden of disease due to respiratory syncytial virus (RSV) infection over time in the Madrid region (Spain): a prospective population-based cohort study
- PMID: 39220460
- PMCID: PMC11361977
- DOI: 10.3389/fpubh.2024.1441786
The effectiveness of nirsevimab in reducing the burden of disease due to respiratory syncytial virus (RSV) infection over time in the Madrid region (Spain): a prospective population-based cohort study
Abstract
Introduction: Respiratory syncytial virus (RSV) infection is one of the main causes of morbidity and mortality from lower respiratory tract infections in children under 5 years of age worldwide. Given that, the objective of this study was estimate the effectiveness of nirsevimab (a single-dose, long-acting, human recombinant monoclonal antibody against RSV) over time for the prevention of respiratory episodes treated at different levels of care.
Methods: A prospective and dynamic population-based cohort study was performed including infants born between April 1 and December 31, 2023, in the Madrid region who resided there during the follow-up period from October 1, 2023, to February 29, 2024. Infants were considered immunized from the day after receiving one dose (50 or 100 mg) of nirsevimab or nonimmunized individuals if they did not receive any dose.
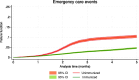
Results: There were 4,100 episodes of primary care, 1,954 hospital emergencies, and 509 admissions, 82 of which required intensive care in the 33,859 participants analyzed. The adjusted effectiveness of nirsevimab in preventing hospitalization due to RSV infection was 93.6% (95% CI: 89.7 to 96.1) at 30 days and 87.6% (95% CI: 67.7 to 95.3) at 150 days. The number needed to treat to prevent one hospitalization were 314.19 (95% CI: 306.22 to 327.99) at 30 days and 24.30 (95% CI: 22.31 to 31.61) at 150 days. The adjusted effectiveness of nirsevimab in avoiding admission to an intensive care unit was 94.4% (95% CI: 87.3 to 97.5) at 30 days and 92.1% (95% CI: 64.0 to 98.3) at 90 days. The adjusted effectiveness of nirsevimab for avoiding primary care consultations and hospital emergency visits was lower.
Discussion: Immunization with nirsevimab is an effective measure for reducing the burden of care related to RSV at all levels of care albeit it decreases throughout follow-up. At 150 days it remained high for preventing hospital admissions. Other articles already published have also demonstrated high effectiveness although with preliminary results, short follow-up periods and wide confidence intervals. None have detected a decrease in effectiveness over time. These results can be quite useful in individual infant prevention and in the design of immunization campaigns.
Keywords: cohort study; effectiveness; hospitalized; infants; intensive care units; nirsevimab; respiratory syncytial virus; respiratory syncytial virus vaccines.
Copyright © 2024 Barbas Del Buey, Íñigo Martínez, Gutiérrez Rodríguez, Alonso García, Sánchez-Gómez, Lasheras Carbajo, Jiménez Bueno, Esteban Vasallo, López Zambrano, Calvo Rey, Sanchez Luna, Molina Olivas and Arce Arnáez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet Lond Engl. (2022) 399:2047–64. doi: 10.1016/S0140-6736(22)00478-0, PMID: - DOI - PMC - PubMed
-
- Wildenbeest JG, Billard MN, Zuurbier RP, Korsten K, Langedijk AC, van de Ven PM, et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med. (2023) 11:341–53. doi: 10.1016/S2213-2600(22)00414-3, PMID: - DOI - PMC - PubMed
-
- Instituto de Salud Carlos III . Red de Vigilancia Epidemiológica. Sistema de Vigilancia de Infección Respiratoria Aguda. Vigilancia centinela de Infección Respiratoria Aguda en Atención Primaria (IRAs) y en Hospitales (IRAG) Gripe, COVID-19 y VRS. Temporada 2022–2023. [Acute Respiratory Infection Surveillance System. Sentinel Surveillance of Acute Respiratory Infection in Primary Care (ARI) and in Hospitals (SARI) Influenza, COVID-19 and RSV. Season 2022–2023]. (2023). Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/...
-
- Dirección General de Salud Pública de la Comunidad de Madrid . Situación epidemiológica del Virus Respiratorio Sincitial (VRS). Comunidad de Madrid. Temporadas (2016). [Epidemiological situation of Respiratory Syncytial Virus (RSV). Madrid region. Seasons 2016/17 to 2022/23]. Available from: https://www.comunidad.madrid/sites/default/files/doc/sanidad/epid/vrs_si...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

