Visible-Light-Induced Dearomative Annulation of Indoles toward Stereoselective Formation of Fused- and Spiro Indolines
- PMID: 39220487
- PMCID: PMC11360027
- DOI: 10.1021/acsomega.4c02848
Visible-Light-Induced Dearomative Annulation of Indoles toward Stereoselective Formation of Fused- and Spiro Indolines
Abstract
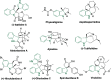
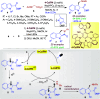
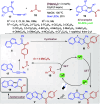
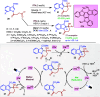
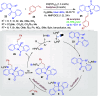
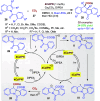
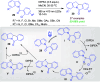
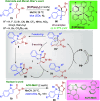
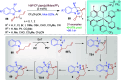
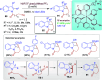
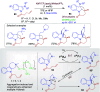
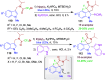
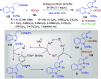
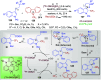
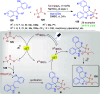
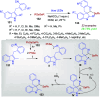
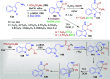
Dearomatization approaches are attractive for their abilities to transform simple, planar arenes into complex, three-dimensional architectures. In particular, visible-light driven dearomatization strategies are significant because of their mild, green, and sustainable nature, enabling the fabrication of new chemical bonds via an electron transfer or energy transfer process. Indole compounds, being potentially bioactive and readily accessible, can be employed efficiently as building blocks for constructing diverse annulated frameworks under photocatalysis. Highly stereoselective radical cascade reactions of appropriate indole systems can provide complex cyclic scaffolds bearing multiple stereocenters. In fact, the past few years have witnessed the renaissance of dearomative cycloadditions of indoles via visible-light-induced photocatalysis. The present review highlights recent advances (2019-mid 2024) in visible-light-driven dearomative annulation of indoles leading to formation of polycyclic indolines, including angularly fused and spiro indolines. Most of the reactions described in this review are simple, providing quick access to the desired products. Additionally, characteristic reaction mechanisms are offered to provide an understand of how indole scaffolds show distinctive reactivity under photocatalytic conditions.
© 2024 The Author. Published by American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures



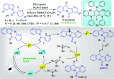
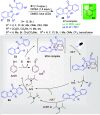
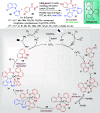


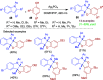




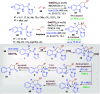
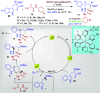

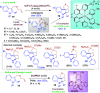
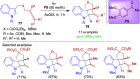










References
-
- Mathada B. S.; Yernale N. G.; Barsha J. N. The Multi-Pharmacological Targeted Role of Indole and its Derivatives: A review. ChemistrySelect 2023, 8, e202204181 10.1002/slct.202204181. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
