Doping Strategy of Monolayer MoS2 to Realize the Monitoring of Environmental Concentration of Desflurane: A First-Principles Study
- PMID: 39220508
- PMCID: PMC11360051
- DOI: 10.1021/acsomega.4c05159
Doping Strategy of Monolayer MoS2 to Realize the Monitoring of Environmental Concentration of Desflurane: A First-Principles Study
Abstract
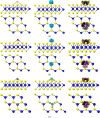
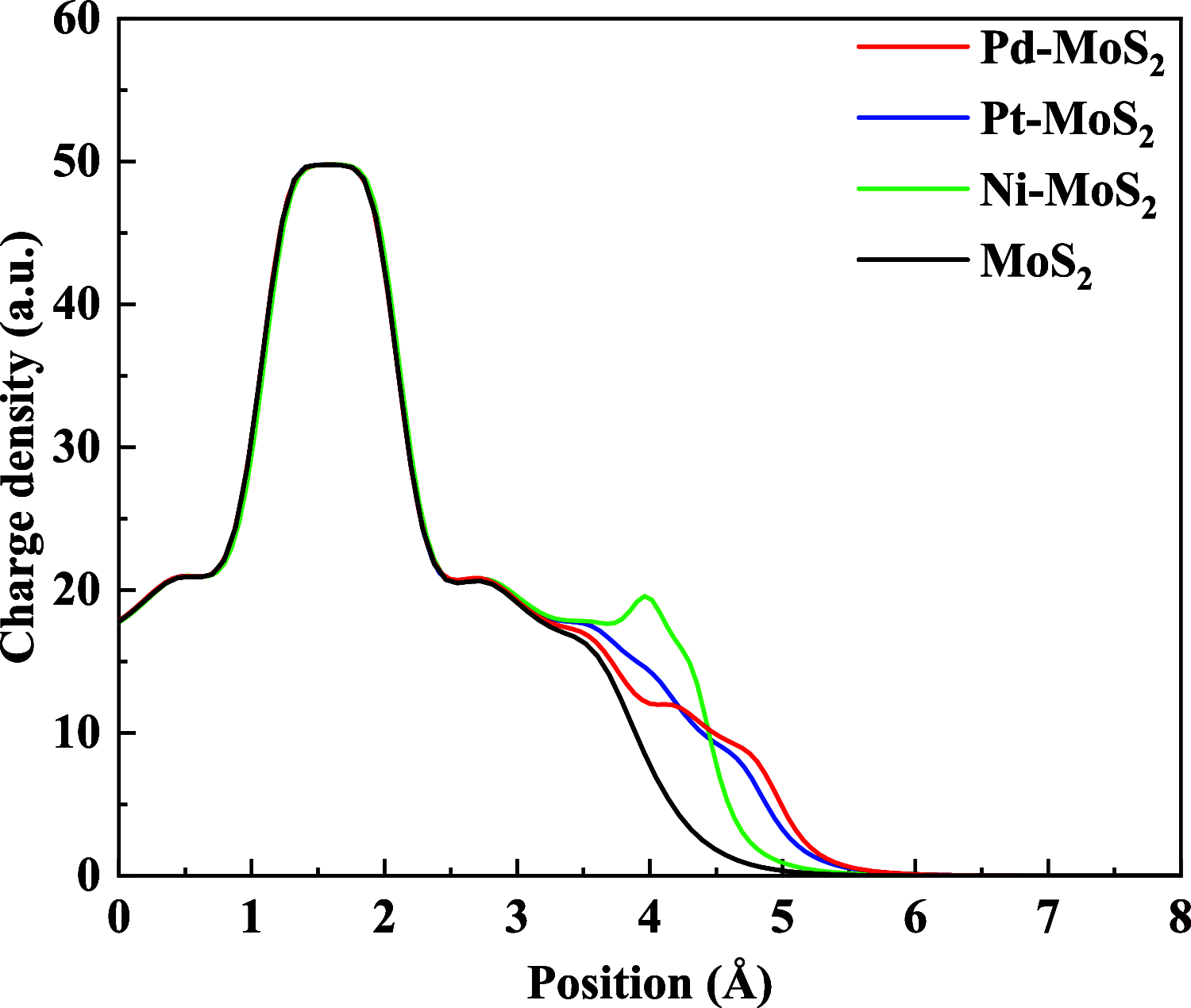
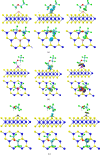
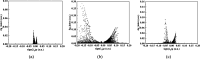
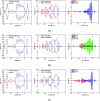
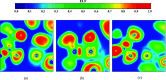
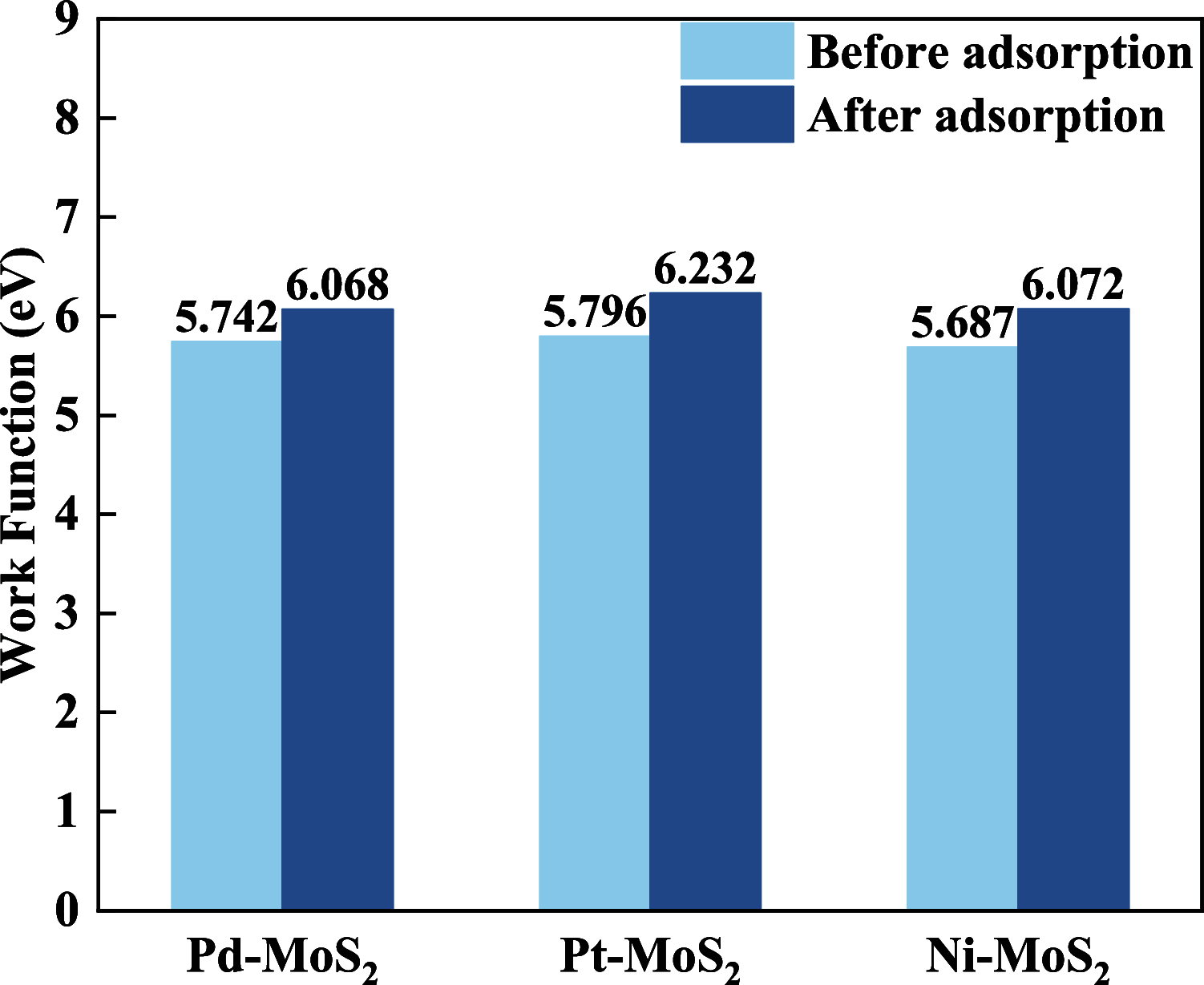
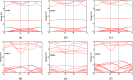
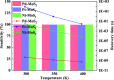
Desflurane is a new volatile inhalation anesthetic that is widely used in medical operation. However, various diseases can be caused by chronic exposure to desflurane, which is also a greenhouse gas. Therefore, it is urgent to find a suitable method for monitoring desflurane. In this paper, the process of doping of Pd, Pt, and Ni on the MoS2 surface is simulated to determine the stability of the doping structure based on first-principles. The adsorption properties and sensing properties of Pd-MoS2, Pt-MoS2, and Ni-MoS2 on desflurane are explored by parameters including independent gradient model based on Hirshfeld partition (IGMH), electron localization function (ELF), and density of states (DOS), sensibility, and recovery time, subsequently. The doping results show that the three doping systems (Pd-MoS2, Pt-MoS2, and Ni-MoS2) are structurally stable, and the chemical bonds are formed with MoS2. The adsorption results show the best chemisorption between Pt-MoS2 and desflurane with the chemical bonds between them. The results of IGMH, ELF, and DOS also confirm it. The sensing characterization results show that the recovery time of Pt-MoS2 ranges between 85.27 and 0.027 s, and the sensitivity ranges from 99.26 to 25.69%, all of which can meet the requirements of the sensor. Considering the adsorption effect and sensing characteristics, Pt-MoS2 can be used as a gas-sensitive material for detecting the concentration of desflurane.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
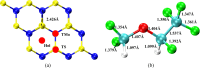



References
LinkOut - more resources
Full Text Sources
