Design, Synthesis, and Antiviral and Fungicidal Activities of 4-Oxo-4 H-quinolin-1-yl Acylhydrazone Derivatives
- PMID: 39220544
- PMCID: PMC11360041
- DOI: 10.1021/acsomega.4c05046
Design, Synthesis, and Antiviral and Fungicidal Activities of 4-Oxo-4 H-quinolin-1-yl Acylhydrazone Derivatives
Abstract
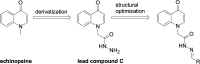
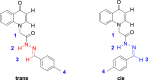
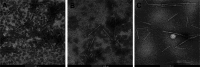
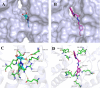
To discover novel antiviral agents, based on the high antiviral activity of (4-oxo-4H-quinolin-1-yl)-acetic acid hydrazide (C), a series of 4-oxo-4H-quinoline acylhydrazone derivatives were designed, synthesized, and first evaluated for their antiviral and fungicidal activities. Most acylhydrazone derivatives exhibited moderate to good antiviral activities in vivo. The inactive, curative, and protective activities of compounds 4 (51.2, 47.6, and 46.3%), 11 (49.6, 43.0, and 45.2% at 500 mg/L), and 17 (47.1, 49.2, and 44.1%) were higher than those of ribavirin (39.2, 38.0, and 40.8%) at 500 mg/L. Molecular docking showed that compound 4 exhibited a stronger affinity to TMV coat protein (TMV-CP) than ribavirin, with a binding energy (-6.89 kcal/mol) slightly lower than that of ribavirin (-6.08 kcal/mol). Microscale thermophoresis showed that compound 4 (K d = 0.142 ± 0.060 μM) exhibited a strong binding ability to TMV-CP, superior to that of ribavirin (K d = 0.512 ± 0.257 μM). The results of transmission electron microscopy showed that compound 4 hindered the self-assembly and growth of TMV. The antifungal activities of most compounds were moderate at 50 mg/L, among which compounds 12 and 21 exhibited a 72.1 and 76.5% inhibitory rate against Physalospora piricola, respectively. Meanwhile, compound 16 exhibited a 60% inhibitory rate against Cercospora arachidicola Hori at 50 mg/L.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Structural Optimization of the Natural Product: Discovery of Almazoles C-D and Their Derivatives as Novel Antiviral and Anti-phytopathogenic Fungus Agents.J Agric Food Chem. 2022 Dec 21;70(50):15693-15702. doi: 10.1021/acs.jafc.2c05898. Epub 2022 Dec 8. J Agric Food Chem. 2022. PMID: 36479881
-
Discovery of Cysteine and Its Derivatives as Novel Antiviral and Antifungal Agents.Molecules. 2021 Jan 13;26(2):383. doi: 10.3390/molecules26020383. Molecules. 2021. PMID: 33450940 Free PMC article.
-
New chalcone derivatives: synthesis, antiviral activity and mechanism of action.RSC Adv. 2020 Jun 26;10(41):24483-24490. doi: 10.1039/d0ra03684f. eCollection 2020 Jun 24. RSC Adv. 2020. PMID: 35516226 Free PMC article.
-
Discovery of Novel α-Methylene-γ-Butyrolactone Derivatives Containing Vanillin Moieties as Antiviral and Antifungal Agents.J Agric Food Chem. 2022 Aug 24;70(33):10316-10325. doi: 10.1021/acs.jafc.2c03632. Epub 2022 Aug 12. J Agric Food Chem. 2022. PMID: 35960686
-
Application of "Hydrogen-Bonding Interaction" in Drug Design. Part 2: Design, Synthesis, and Structure-Activity Relationships of Thiophosphoramide Derivatives as Novel Antiviral and Antifungal Agents.J Agric Food Chem. 2015 Nov 4;63(43):9435-40. doi: 10.1021/acs.jafc.5b02676. Epub 2015 Oct 26. J Agric Food Chem. 2015. PMID: 26485246 Review.
Cited by
-
Chemoselective synthesis of tunable poly-functionalized binary pyrazolyl and annulated pyrazolo/pyrido anchored on quinolinone: insecticidal and antioxidant studies.RSC Adv. 2025 Feb 24;15(8):6050-6067. doi: 10.1039/d4ra08834d. eCollection 2025 Feb 19. RSC Adv. 2025. PMID: 39995463 Free PMC article.
References
-
- Gan X. H.; Hu D. Y.; Li P.; Wu J.; Chen X. W.; Xue W.; Song B. A. Design, synthesis, antiviral activity and three-dimensional quantitative structure-activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety: Antiviral activity of novel 1,4-pentadien-3-onederivatives containing the 1,3,4-oxadiazole moiety. Pest Manag. Sci. 2016, 72, 534–543. 10.1002/ps.4018. - DOI - PubMed
-
- Chi Y.; He H. W.; Chen C. Y.; Zhao S. Y.; Zhou H.; Xu D.; Liu X. L.; Xu G. Furofuran lignans for plant protection: discovery of sesamolin and its derivatives as novel anti-tobacco mosaic virus and antibacterial agents. J. Agric. Food Chem. 2023, 71, 10798–10808. 10.1021/acs.jafc.3c03257. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
