Sulforaphane's Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-Dependent and -Independent Mechanism of Anti-SARS-CoV-2 Activity
- PMID: 39220635
- PMCID: PMC11360660
- DOI: 10.35534/jrbtm.2024.10010
Sulforaphane's Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)-Dependent and -Independent Mechanism of Anti-SARS-CoV-2 Activity
Abstract
It is well established that Nrf2 plays a crucial role in anti-oxidant and anti-inflammatory functions. However, its antiviral capabilities remain less explored. Despite this, several Nrf2 activators have demonstrated anti-SARS-CoV-2 properties, though the mechanisms behind these effects are not fully understood. In this study, using two mouse models of SARS-CoV-2 infection, we observed that the absence of Nrf2 significantly increased viral load and altered inflammatory responses. Additionally, we evaluated five Nrf2 modulators. Notably, epigallocatechin gallate (EGCG), sulforaphane (SFN), and dimethyl fumarate (DMF) exhibited significant antiviral effects, with SFN being the most effective. SFN did not impact viral entry but appeared to inhibit the main protease (MPro) of SARS-CoV-2, encoded by the Nsp5 gene, as indicated by two protease inhibition assays. Moreover, using two Nrf2 knockout cell lines, we confirmed that SFN's antiviral activity occurs independently of Nrf2 activation in vitro. Paradoxically, in vivo tests using the MA30 model showed that SFN's antiviral function was completely lost in Nrf2 knockout mice. Thus, although SFN and potentially other Nrf2 modulators can inhibit SARS-CoV-2 independently of Nrf2 activation in cell models, their Nrf2-dependent activities might be crucial for antiviral defense under physiological conditions.
Keywords: Lung; MA30; MPro; Nrf2; SARS-CoV-2; Sulforaphane.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in This paper.
Figures
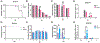
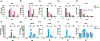
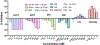
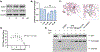

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
