Phillygenin inhibits neuroinflammation and promotes functional recovery after spinal cord injury via TLR4 inhibition of the NF-κB signaling pathway
- PMID: 39220679
- PMCID: PMC11363727
- DOI: 10.1016/j.jot.2024.07.013
Phillygenin inhibits neuroinflammation and promotes functional recovery after spinal cord injury via TLR4 inhibition of the NF-κB signaling pathway
Abstract
Background: Spinal cord injuries (SCIs) trigger a cascade of detrimental processes, encompassing neuroinflammation and oxidative stress (OS), ultimately leading to neuronal damage. Phillygenin (PHI), isolated from forsythia, is used in a number of biomedical applications, and is known to exhibit anti-neuroinflammation activity. In this study, we investigated the role and mechanistic ability of PHI in the activation of microglia-mediated neuroinflammation and subsequent neuronal apoptosis following SCI.
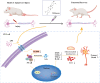
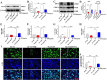
Methods: A rat model of SCI was used to investigate the impact of PHI on inflammation, axonal regeneration, neuronal apoptosis, and the restoration of motor function. In vitro, neuroinflammation models were induced by stimulating microglia with lipopolysaccharide (LPS); then, we investigated the influence of PHI on pro-inflammatory mediator release in LPS-treated microglia along with the underlying mechanisms. Finally, we established a co-culture system, featuring microglia and VSC 4.1 cells, to investigate the role of PHI in the activation of microglia-mediated neuronal apoptosis.
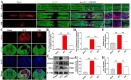
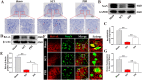
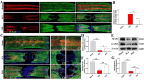
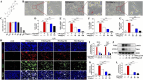
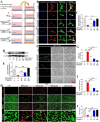
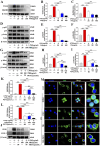
Results: In vivo, PHI significantly inhibited the inflammatory response and neuronal apoptosis while enhancing axonal regeneration and improving motor function recovery. In vitro, PHI inhibited the release of inflammation-related factors from polarized BV2 cells in a dose-dependent manner. The online Swiss Target Prediction database predicted that toll-like receptor 4 (TLR4) was the target protein for PHI. In addition, Molecular Operating Environment software was used to perform molecular docking for PHI with the TLR4 protein; this resulted in a binding energy interaction of -6.7 kcal/mol. PHI inhibited microglia-mediated neuroinflammation, the production of reactive oxygen species (ROS), and activity of the NF-κb signaling pathway. PHI also increased mitochondrial membrane potential (MMP) in VSC 4.1 neuronal cells. In BV2 cells, PHI attenuated the overexpression of TLR4-induced microglial polarization and significantly suppressed the release of inflammatory cytokines.
Conclusion: PHI ameliorated SCI-induced neuroinflammation by modulating the TLR4/MYD88/NF-κB signaling pathway. PHI has the potential to be administered as a treatment for SCI and represents a novel candidate drug for addressing neuroinflammation mediated by microglial cells.
The translational potential of this article: We demonstrated that PHI is a potential drug candidate for the therapeutic management of SCI with promising developmental and translational applications.
Keywords: NF-κB signaling pathway; Neuroinflammation; Neuronal apoptosis; Phillygenin; Spinal cord injury.
© 2024 The Authors.
Conflict of interest statement
The authors declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

