Signaling Modulation Mediated by Ligand Water Interactions with the Sodium Site at μOR
- PMID: 39220695
- PMCID: PMC11363324
- DOI: 10.1021/acscentsci.4c00525
Signaling Modulation Mediated by Ligand Water Interactions with the Sodium Site at μOR
Abstract
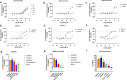
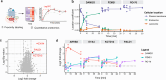
The mu opioid receptor (μOR) is a target for clinically used analgesics. However, adverse effects, such as respiratory depression and physical dependence, necessitate the development of alternative treatments. Recently we reported a novel strategy to design functionally selective opioids by targeting the sodium binding allosteric site in μOR with a supraspinally active analgesic named C6guano. Presently, to improve systemic activity of this ligand, we used structure-based design, identifying a new ligand named RO76 where the flexible alkyl linker and polar guanidine guano group is swapped with a benzyl alcohol, and the sodium site is targeted indirectly through waters. A cryoEM structure of RO76 bound to the μOR-Gi complex confirmed that RO76 interacts with the sodium site residues through a water molecule, unlike C6guano which engages the sodium site directly. Signaling assays coupled with APEX based proximity labeling show binding in the sodium pocket modulates receptor efficacy and trafficking. In mice, RO76 was systemically active in tail withdrawal assays and showed reduced liabilities compared to those of morphine. In summary, we show that targeting water molecules in the sodium binding pocket may be an avenue to modulate signaling properties of opioids, and which may potentially be extended to other G-protein coupled receptors where this site is conserved.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): S.M. is a cofounder of Sparian Biosciences. R.O.D. holds equity in Septerna, Inc. Other authors declare no competing financial interest.
Figures





References
-
- Pasternak G. W.; Childers S. R.; Pan Y.-X., Emerging Insights into Mu Opioid Pharmacology. In Substance Use Disorders: From Etiology to Treatment; Nader M. A.; Hurd Y. L., Eds.; Springer International Publishing: Cham, 2020; pp 89–125. - PubMed
-
- National Center for Health Statistics . VSRR Provisional Drug Overdose Death Counts. Available from https://data.cdc.gov/d/xkb8-kh2a.
-
- Eans S. O.; Ganno M. L.; Mizrachi E.; Houghten R. A.; Dooley C. T.; McLaughlin J. P.; Nefzi A. Parallel Synthesis of Hexahydrodiimidazodiazepines Heterocyclic Peptidomimetics and Their in Vitro and in Vivo Activities at μ (MOR), δ (DOR), and κ (KOR) Opioid Receptors. J. Med. Chem. 2015, 58 (12), 4905–4917. 10.1021/jm501637c. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
